Keywords
Hypersensitivity, allergy, allergic reaction, physiomesh, hernia mesh, case report
Hypersensitivity, allergy, allergic reaction, physiomesh, hernia mesh, case report
Ventral hernia repair is a common surgical procedure1. Physiomesh® Flexible Composite Mesh (Ethicon, Somerville, NJ, USA) was recalled in May 2016, due to a higher rate of recurrence and reoperations following laparoscopic ventral hernia repair compared with other meshes (https://archive.org/details/EthiconPhysiomeshRecall).
In this case report, we describe a rare complication of using Physiomesh®. Mesh-related allergic reaction following hernia repair has only been described in one previous case report2. We present a patient with allergic reaction to Physiomesh® after laparoscopic ventral hernia repair.
The patient was a 50-year-old male. Ten years prior, the patient had a primary umbilical open non-mesh repair with good effect. The patient’s father died of colon cancer. There were no other dispositions. In 2014, the patient had recurrence of the hernia and underwent a laparoscopic intraperitoneal onlay mesh (IPOM) reoperation with insertion of Physiomesh®. The hernia defect, measuring 2.5 cm, was sutured prior to application of a Physiomesh®, measuring 13×13 cm and placed with IPOM technique and fixated with ProTack® (Medtronic/Covidien, CT, USA) with double crown tacks. An emergency operation was performed later the same day due to severe abdominal pain reported by the patient. At the operation, no perforation, bleeding, or pathology was found. The surgeon removed three tacks at the point of maximum pain. The patient received intravenous (i.v) cefuroxime 1.5 g × 2 perioperatively and by oral administration ibuprofen (600 mg × 4) and paracetamol PRN. He was discharged two days later with regained bowel activity and no pain.
Two months later, the patient was readmitted with acute epigastric pain. Blood tests showed leucocytes 19×109/l and CRP 140 mg/l, with no eosinophilia. An abdominal CT scan was performed, due to suspicion of perforated ulcer. The CT showed signs of ileus with no obvious obstruction or recurrence of the hernia. The patient underwent diagnostic laparoscopy with conversion to laparotomy, due to insufficient overview. The proximal half of the small intestine was dilated with no sign of obstruction or adhesions. The retroperitoneum was edematous, increasing towards the mesh. A thick layer of fibrin was observed in close proximity to the mesh, and the peritoneum was covered by a hard layer of connective tissue, which resembled granulation tissue. Mesh and tacks were removed, and tissue samples were sent for histology. Histology showed inflammation with an allergic foreign body reaction (Figure 1). The tissue samples consisted of soft tissue with chronic inflammation, many infiltrating eosinophils, fibrinoid degeneration, fibrosis, and areas resembling granulation tissue. Scattered birefringent foreign body material was present but there were no giant cells or granulomas. Postoperatively, the patient was treated with analgesics and a nasogastric tube due to pain and nausea, respectively. The patient was discharged 2.5 weeks after his reoperation with normalized bowel function and no pain.
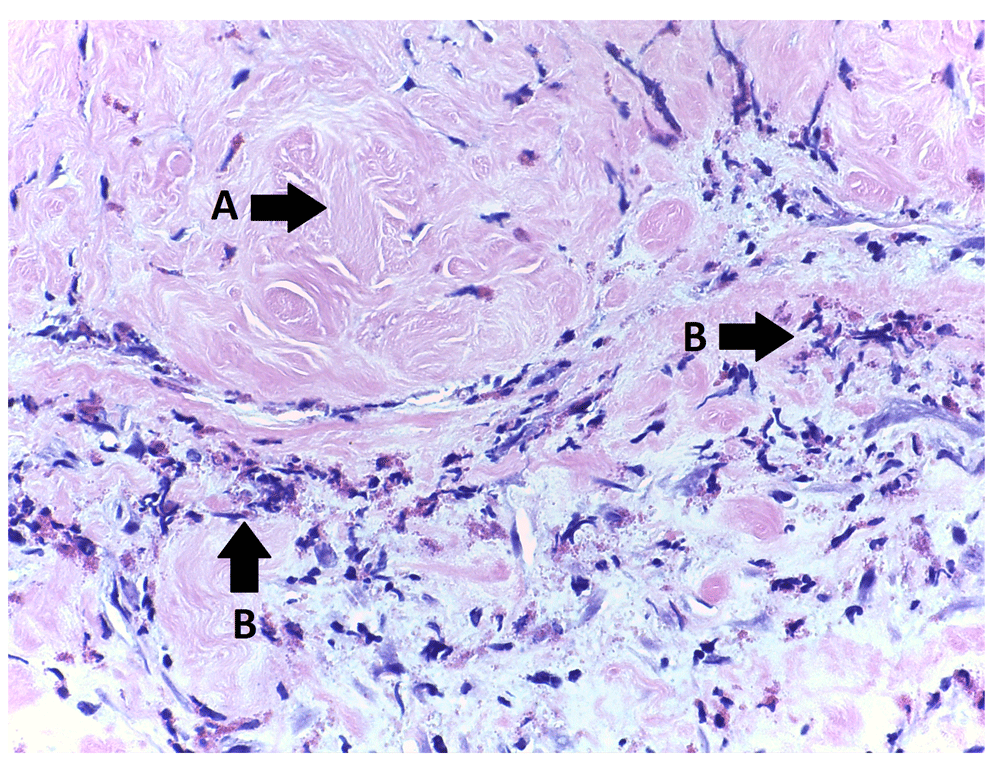
H&E stained tissue sample taken from peritoneum in close proximity to the Physiomesh®. The histology showed inflammation with an allergic foreign body reaction. Arrow A indicates hyaline fibrosis; arrows B indicates eosinophils, some intact and some degranulated.
Two weeks later, the patient was readmitted with incomplete ileus. CT showed the small bowel with a thickened wall and gathered in a conglomerate. Small bowel follow-through indicated normal passage. The patient was not operated on and was well and discharged four days later. Six months after the mesh was removed, the patient had CT-diagnosed recurrence of his umbilical hernia, as well as a new incisional hernia. The patient did not want another operation, and the hernias were therefore relieved with a truss. Eleven months after removing the mesh, the patient was again admitted with incomplete ileus and was treated non-operatively. Due to strong pain from the umbilical and incisional hernias, the patient had a final surgical procedure 18 months after removal of the Physiomesh®. The hernia defect now measured 10 cm craniocaudally and 8 cm transversely. The surgical procedure was performed as an open repair. The small intestines were adherent to the hernia sac and were only covered by skin. Complete adhesiolysis was performed, and the hernia was repaired with a modified Stoppa procedure with a Progrip™ mesh (Medtronic/Covidien, CT, USA) in the sublay position. During admission, the patient received standard perioperative antibiotics and analgesics. No further treatment was given. Five days later, the patient was well and discharged. For follow-up, the patient was contacted once by telephone. One year after this final operation, there have been no further admissions to hospital.
The focus on foreign body implantation to the abdominal cavity has increased. This is partly due to the recall of Physiomesh® Flexible Composite Mesh on the basis of high rates of recurrence, as well as a recently published study on mesh-related surgical complications3.
We have presented a rare case of allergic reaction to Physiomesh®. The clinical presentation was acute epigastric pain two months after a laparoscopic ventral hernia repair. CT showed ileus with no sign of obstruction or recurrent hernia. The diagnosis of allergic reaction to the mesh was finally confirmed by histological examination of peritoneal tissue samples with chronic inflammation, many infiltrating eosinophils, fibrinoid degeneration, fibrosis, and areas resembling granulation tissue.
The strengths in the approach to this case were that the surgeons, based on the patient history and the perioperative findings, suspected an allergic reaction. They did so despite the rarity of allergic reaction and no indication from paraclinical tests. Furthermore, based on their assessment, they removed the mesh and took out tissue samples for histological verification. The limitation in the approach to this case was that the allergic reaction was not recognized at the first operation following mesh insertion and the patient had to wait for two months and develop incomplete ileus before the hypersensitivity reaction was diagnosed and the mesh removed.
Hypersensitivity reactions are immune responses that cause tissue injury4. Type IV hypersensitivity is the T cell-mediated response, which for instance is involved in contact sensitivity, chronic inflammation, and graft rejection4. There are few published reports on hypersensitivity reactions to meshes in general and only one regarding a mesh used for a hernia repair2. There are, to the best of our knowledge, no previously published articles reporting hypersensitivity to Physiomesh®. However, an adverse event report has been filed at the U.S. Food and Drug Administration regarding a possible allergic reaction (https://archive.org/details/MAUDEAdverseEventReportETHICONINC). Physiomesh® is a polypropylene mesh encapsulated by polydioxanone and coated with a monocryl layer on both sides of the mesh5.
In the present case report, the indications that a component of the Physiomesh® was the cause of the allergic reaction were that the edema and the fibrin layer were most pronounced in close proximity to the mesh, that the patient experienced remission of his symptoms after removal of the mesh, and that the patient experienced no hypersensitivity to the monofilament polyester mesh that was used for the final sublay repair.
We have presented a very rare case of severe allergic reaction to Physiomesh®. This adverse event adds to the risk of complications following implantation of mesh to reinforce a hernia repair. The allergic reaction was not suspected until during operation, since paraclinical tests were not indicative of allergy. Therefore, this case also highlights the importance of surgeons’ clinical assessment and ability to take relevant action, which in this case consisted of mesh removal and retrieving tissue samples for histology.
Written informed consent for publication of the patient’s clinical details and/or clinical images was obtained from the patient.
All authors contributed to the acquisition of the data. LQM interpreted the data and drafted the manuscript. TB, TPH and JR revised the manuscript critically. All authors have approved the final manuscript and are accountable for the work.
LQM, TB and TPH have nothing to disclose. JR reports grants from Johnson & Johnson, grants and personal fees from Bard, personal fees from Merck, outside the submitted work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Köckerling F, Simon T, Hukauf M, Hellinger A, et al.: The Importance of Registries in the Postmarketing Surveillance of Surgical Meshes.Ann Surg. 2017. PubMed Abstract | Publisher Full TextCompeting Interests: Workshop honorarium by Bard and Tutogen
Reviewer Expertise: Mesh technology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: Research Grant by Bard; Honorarium by Springer
Reviewer Expertise: Surgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 May 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)