Introduction
At the turn of the 20th century, bacterial meningitis was an almost universally fatal disease. Two important medical advances—antibiotics and vaccination—have dramatically decreased the incidence and the case fatality rate of bacterial meningitis, particularly within pediatric populations. Some of the pathogens that caused meningitis 20 years ago are now more likely to be encountered by medical trainees in reviewing textbooks than in clinical practice. With these shifting dynamics, a greater understanding of the current epidemiology of community-acquired meningitis is needed. In addition, several pathways involved in the pathogenesis of bacterial meningitis have been elucidated. We review some of these models and provide an update on the role of the microbiome in the development of meningitis.
Overview of the epidemiology of bacterial meningitis in childhood
Traditional descriptions of bacterial meningitis in childhood have stratified causative pathogens on the basis of age, as there is a stark contrast in the bacterial pathogens that cause meningitis in newborns compared with older children. Meningitis in children older than 60 days, called “pediatric bacterial meningitis” in this review, is often caused by encapsulated bacteria that colonize the nasopharynx and other body sites. Meningitis in children younger than 60 days, called “young infant bacterial meningitis” in this review, is further stratified by gestational age and timing of onset of infection1. In general, infections that occur within the first 7 days of life of a term neonate are described as early onset disease, whereas infections occurring from 7 to 60 days after birth are described as late-onset disease1. Early onset disease is caused predominantly by bacteria transmitted at the time of parturition, whereas late-onset disease is caused by members of the microbiome transmitted at birth or through exposures after birth, such as maternal contact or method of feeding1–5. Despite the distinctions in pathogens between the age cohorts, pathogens of pediatric bacterial meningitis can also cause disease in young infants and vice versa.
Pediatric bacterial meningitis
For over 30 years, the Centers for Disease Control and Prevention (CDC) in the US has published surveillance data of bacterial meningitis. In 1995, the CDC established the Active Bacterial Core Surveillance, an active monitoring system for invasive pathogens, and since then has made annual reports available to the public (http://www.cdc.gov/abcs/reports-findings/surv-reports.html). The epidemiology of meningitis in the US has profoundly changed over the past several decades. In 1978–1981, Haemophilus influenzae was the most frequent cause of meningitis (48.3% of cases), followed by Neisseria meningitidis (19.6%) and Streptococcus pneumoniae (13.3%)6. By 2014, in children under age 5 in the US, S. pneumoniae was the most frequently identified pathogen whereas H. influenzae was rarely detected7.
Although S. pneumoniae is the most frequent etiology of bacterial meningitis in the US, the incidence of pneumococcal meningitis has dramatically decreased over the past two decades because of the implementation of pneumococcal serotype vaccines (Figure 1a)8. In 2000, a seven-valent pneumococcal vaccine (PCV7) targeting a subset of serotypes associated with invasive disease was licensed in the US8. After the introduction of PCV7, the rate of invasive disease caused by PCV7 serotypes fell from 80 per 100,000 population in 2000 to below one per 100,000 population in 20079. Meningitis caused by PCV7 serotypes also significantly decreased, from 8.2 cases per 100,000 in 1998–1999 to 0.59 cases per 100,000 in 2004–200510. Substantial reductions in invasive pneumococcal infections were also observed in other countries after implementation of the PCV7 vaccine11,12. However, surveillance data identified a rise in the incidence of meningitis caused by serotypes not included in the vaccine, known as serotype replacement13, prompting the development of a 13-valent pneumococcal vaccine (PCV13) licensed in the US in 2010. Since the implementation of PCV13 in several countries, continued decreases in invasive S. pneumoniae diseases have been observed (Figure 1a)14–20. However, there are conflicting data as to whether the introduction of PCV13 has decreased the rate of S. pneumoniae meningitis20–22.
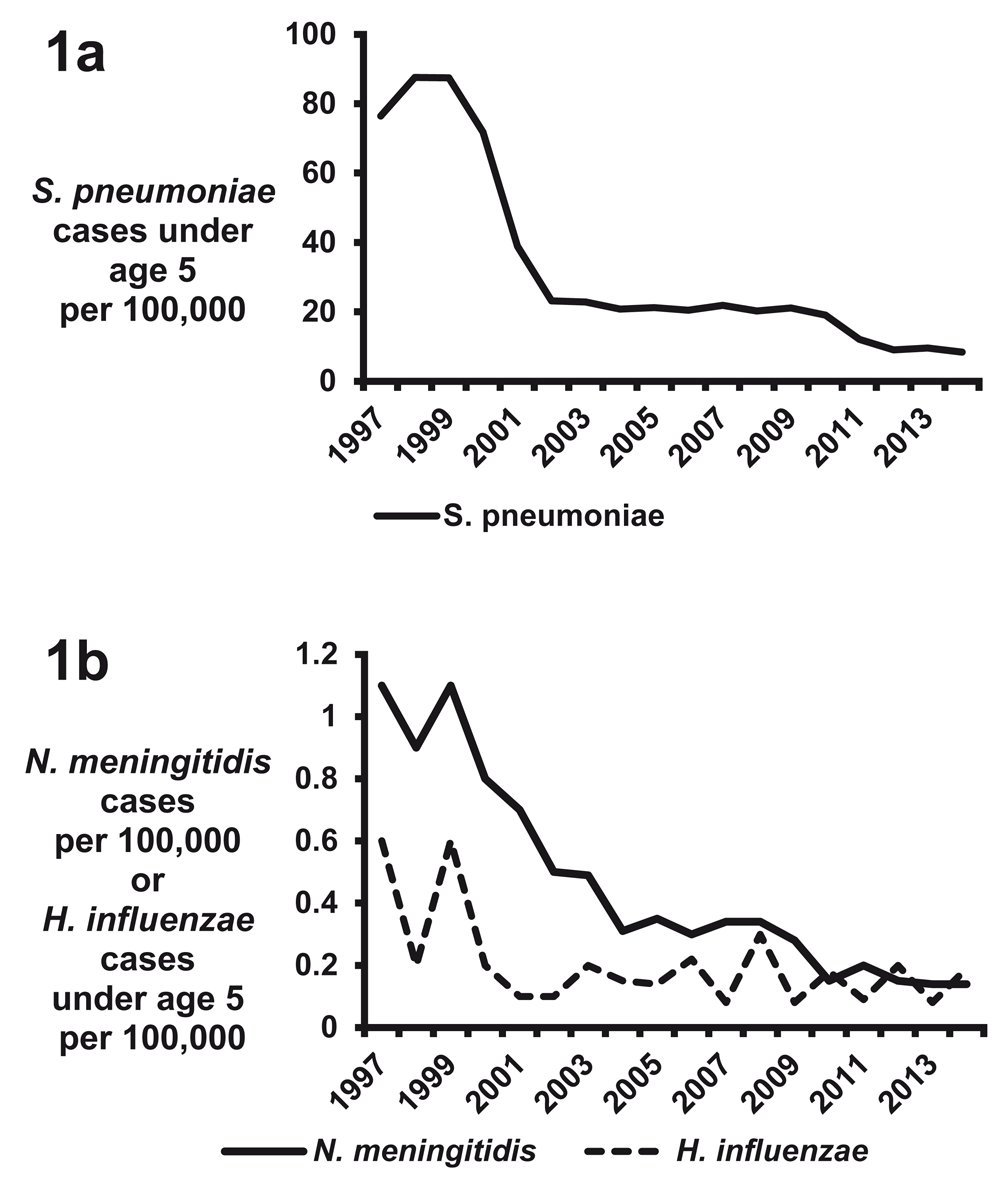
Figure 1.
Rates of (a) invasive disease for Streptococcus pneumoniae in children under the age of 5 and (b) invasive disease caused by Haemophilus influenzae in children under the age of 5 and by Neisseria meningitides in all ages. All data are from accumulated Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance reports 1997–2014 (http://www.cdc.gov/abcs/reports-findings/surv-reports.html).
The dramatic reduction in the incidence of H. influenzae meningitis demonstrates the tremendous success of the serotype B vaccine23. H. influenzae type B is a highly virulent strain that caused the majority of H. influenzae meningitis cases23,24. In 1978–1981, the peak incidence of H. influenzae meningitis was in infants 9 to 11 months of age, and the attack rate was 70 cases of meningitis per 100,000 population per year6. In the 1980s, several H. influenzae type B vaccines were in development; among them were an unconjugated polysaccharide formulation licensed in the US in 1985 and the conjugate vaccine licensed in the US in 1987. Implementation of the vaccines caused a rapid decline in H. influenzae disease; by 2014, the CDC identified only 40 invasive cases of H. influenzae type B infection, representing an invasive disease rate of 0.19 per 100,000 US children under the age of 5 (Figure 1b)25–27. Similar analyses in other countries have also demonstrated a significant reduction of H. influenzae meningitis after implementation of the type B vaccine11,12,28.
For N. meningitidis, 13 serogroups are currently known and only six serogroups are recognized to cause meningitis (A, B, C, W-135, X, and Y)29. During 1978–1981 in the US, the highest rate of N. meningitidis-associated meningitis was in children aged 3 to 5 months, with over 10 cases per 100,000 population per year6. Over the next 25 years, the incidence of meningitis caused by N. meningitidis decreased in the US and this was hypothesized to be due to a combination of environmental, organism, and host factors (Figure 1b)30. In 2005, the meningococcal conjugate quadrivalent vaccine (MenACWY) targeting serogroups A, C, Y, and W-135 was licensed for use in adolescents in the US. Further reductions in disease have been observed after implementation of the vaccine; in 2014, meningococcemia occurred in 0.14 cases per 100,000 persons in the US, representing a total of 443 invasive cases27. Infants under a year of age have the highest incidence of meningitis from N. meningitidis; an estimated 2.74 cases of meningitis per 100,000 occurred in the US from 2006 to 201231. From that same study, serogroup B caused 64% of cases of meningitis, serogroup Y caused 16%, and serogroup C caused 12%31. Similar reductions in N. meningitidis-associated diseases have been observed in other countries after the implementation of vaccination strategies12,32,33. N. meningitidis is a well-known cause of meningitis epidemics in the sub-Saharan region of Africa, where attack rates are as high as 800 cases per 100,000 population34,35. Serogroup A accounts for 80–85% of all outbreak cases, and many global efforts in distributing vaccines to epidemic regions of Africa have significantly reduced the incidence of meningitis35,36.
Young infant bacterial meningitis
In older studies, Streptococcus agalactiae (Group B Streptococcus, or GBS) was the most frequently identified pathogen from cases of young infant bacterial meningitis, followed in incidence by other organisms, including Escherichia coli and Listeria monocytogenes37–42. In a 2014 study from the UK and Ireland, GBS remained the most common cause of meningitis despite interventions to reduce disease caused by this organism43. This result contrasts with a 2014 study of young infants in California, where E. coli was the most frequently identified pathogen in meningitis44. Other recent studies have described the importance of enteric Gram-negative organisms causing meningitis in this age group, while the incidence of meningitis caused by L. monocytogenes has decreased44–46.
The primary reason behind the shift in the epidemiology in the US has been the implementation of intrapartum antibiotic prophylaxis against GBS39,47,48. The CDC, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists published unified prophylaxis guidelines in 1996, screening guidelines in 2002, and revised guidelines in 201049–55. Pregnant mothers are screened for rectovaginal colonization at 35 to 37 weeks’ gestation for GBS, and colonized mothers are provided with intrapartum antibiotic prophylaxis52–54. Additionally, intrapartum antibiotics are indicated for mothers if they have a previous infant with invasive GBS disease, history of GBS bacteriuria, or unknown GBS status with at least one of the following: delivery at less than 37 weeks’ gestation, amniotic membrane rupture of at least 18 hours, fever, or an intrapartum nucleic acid amplification test (NAAT) positive for GBS55. Efficacy of reducing transmission is enhanced if a beta-lactam or cephalosporin antibiotic is given at least 4 hours prior to delivery56,57. This intervention has dramatically reduced the rates of early onset sepsis from GBS, including meningitis (Figure 2a)48. However, intrapartum prophylaxis has not reduced the rate of late-onset GBS invasive disease (Figure 2a)48,58,59. Multiple factors likely contribute to the unchanged incidence of GBS late-onset disease, as intrapartum antibiotics reduce but do not abrogate GBS colonization, and transmission of GBS after birth may also occur through maternal, nosocomial, or environmental contacts3,59.
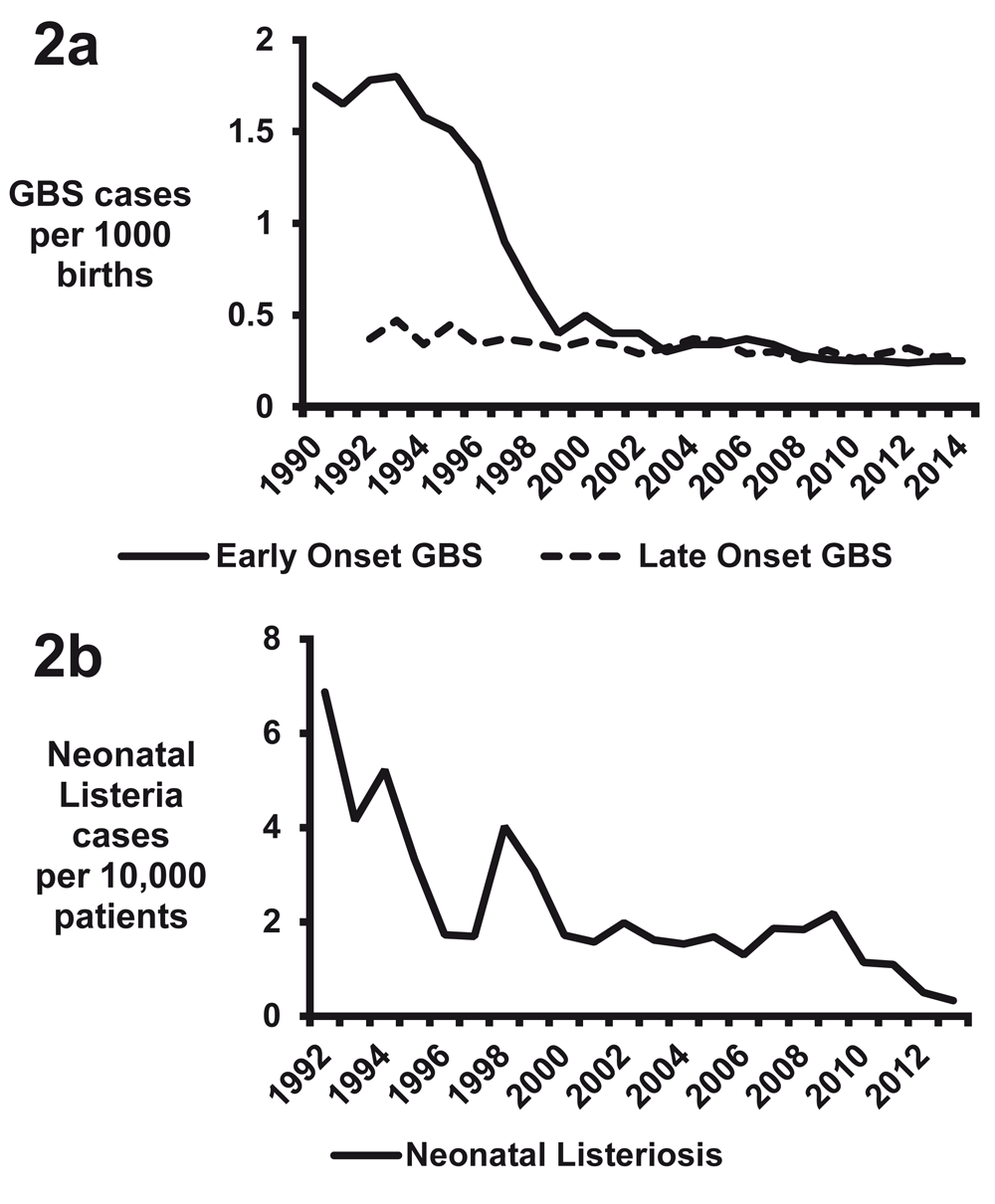
Figure 2. Rates of invasive young infant infections in the United States.
(a) Early onset Group B Streptococcus (GBS) disease data for 1990 to 1998 are from 47 and for 1999 to 2014 are from accumulated Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance reports. Late-onset GBS disease data for 1992–2005 are from 59 and for 2006–2014 are from accumulated CDC Active Bacterial Core surveillance reports. (b) Listeria data are from 82.
The overall incidence of meningitis and sepsis from E. coli has remained relatively stable in term infants since the implementation of GBS prophylaxis guidelines in the US and France60–64. However, trends of increasing frequency of disease in select subgroups have been described, including an increase of E. coli early onset sepsis in preterm or very-low-birth-weight neonates1,64–66. The incidence of late-onset disease from E. coli has also increased in term or preterm infants1,64–66. E. coli isolated from cases of meningitis is frequently resistant to ampicillin45,60, but an increase in ampicillin-resistant E. coli has been observed only in low-birth-weight or premature infants60,67–69. Recent analyses of the infant gastrointestinal microbiome have identified the presence of many antibiotic-resistance genes, but it is not known why the frequency of resistant E. coli invasive disease has not increased in all young infants during the period of intrapartum prophylaxis70–74.
Previously in the US, L. monocytogenes was a common cause of neonatal meningitis, but in 2014 only 13 cases of meningitis and 37 cases of bacteremia were reported in neonates75. Based on the birth data for the US in 2014 (3,988,076 total births), the 50 cases of neonatal listeriosis would translate to a rate of 1.25 invasive cases per 100,000 births. This is in stark contrast to 17.4 invasive cases per 100,000 births in 1989, which decreased to 8.6 cases per 100,000 births by 199376. Increased safety in food product handling was the major driving force in the reduction of cases in the US during the 1980s and 1990s77–79. By 2004–2009, seven cases per 100,000 births on average were complicated by L. monocytogenes infection, according to estimates calculated by using data from Silk et al.80 and the US birth rate81. The GBS intrapartum prophylaxis recommendations may have contributed to the reduction of listeriosis, as the primary antibiotics used for prophylaxis—penicillin G and ampicillin—have excellent activity against L. monocytogenes82. Supporting this hypothesis are reduced rates of invasive disease in infants under 30 days of life identified from the Pediatric Health Information System, a database that uses pediatric discharge data from 45 tertiary pediatric hospitals in the US82. A total of 6.87 listeria cases per 10,000 patients occurred in 1992 compared with 0.33 cases per 10,000 patients in 2013, and this correlated with the reduction in GBS invasive diseases (Figure 2b)82.
Pathogenesis of meningitis
The pathogenesis of bacterial meningitis is often characterized by four primary processes: (1) colonization of the epithelial barrier, (2) entrance into the circulatory system, (3) breeching of the blood-brain barrier (BBB), and (4) central nervous system (CNS) inflammation and injury (Figure 3)83–86.
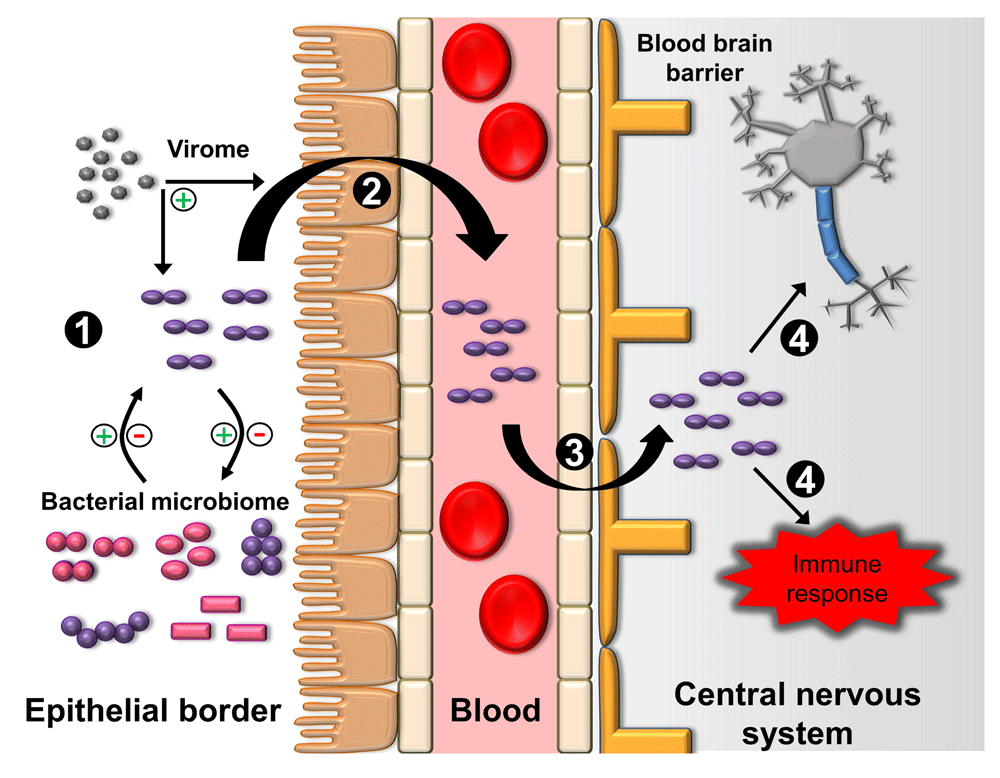
Figure 3. Four generalized steps are involved in the pathogenesis of bacterial meningitis.
(1) Bacterial colonization of the epithelial border. Colonization is affected by the host and other members of the microbiome, including bacteria and viruses (virome). Bacteria may have synergistic or antagonistic effects on colonization, while viruses may enhance colonization. (2) Bacterial invasion of the epithelial surface into the bloodstream. This process can be enhanced by viruses. (3) Bacterial breeching of the blood-brain barrier. Various pathways have been described in the penetration of the blood-brain barrier, including transcellular, paracellular, and “Trojan horse” mechanisms of entry. (4) Bacterial replication in the central nervous system. The release of bacterial products causes direct toxicity to neurons and stimulation of the immune response, which contributes to additional neurotoxicity.
Epithelial surfaces in humans are the interface in which complex interactions develop among the host, environment, and diverse populations of organisms. S. pneumoniae, H. influenzae, N. meningitidis, and many other bacterial organisms colonize the nasopharyngeal tract, being freely exchanged by aerosolization and contact with secretions87,88. Although these organisms collectively inhabit the nasopharynx, this colonization is not necessarily a peaceful co-inhabitation between organisms; rather it is an evolving balance among mutualism, competition, and outright antagonism. S. pneumoniae can produce hydrogen peroxide that causes a rapid decrease in the growth of H. influenzae89. Likewise, H. influenzae type B can induce an immune response that selectively targets S. pneumoniae while leaving H. influenzae colonies unscathed90,91. Vaccination efforts have also altered the composition of the nasopharyngeal microbiome and have altered the epidemiology of acute otitis media92,93. Ultimately, there are many inter-bacterial interactions in the nasopharynx, and further investigation may reveal compositions of the microbiome that modify the risk of meningitis92,94,95.
Interactions with the host are not limited to the bacterial domain, as co-inhabitation of specific viruses with bacteria led to synergistic relationships88. Viruses can contribute to bacterial adherence to epithelial surfaces through viral factors and upregulation of host adhesion proteins88. Viruses also can contribute to the bacterial invasion of the epithelial surfaces by causing disruption of the epithelial barrier and by impairing the immune response88,96–98. Associations between viral infection and meningitis have been observed, and these associations may partially explain outbreaks or seasonality of meningitis99–103.
Most of the bacterial pathogens of young infant and pediatric meningitis contain a polysaccharide capsule that contributes to invasion of the epithelial surface and survival in the bloodstream48,84,104–109. The capsule is an important virulence factor that confers added protection from phagocytosis, complement pathways, and penetration of the epithelium and BBB48,84,85,110–112. Children with antibody deficiencies, defects of the complement pathway, or asplenia are at particular risk from invasive disease of these pathogens because of their diminished ability to target and clear encapsulated pathogens from the bloodstream113.
Bacterial pathogens most often reach the BBB via the bloodstream. A threshold of bacteremia contributes to the breeching of the BBB, as a higher quantity of bacteria in the bloodstream is associated with increased risk of developing meningitis104,114–117. Various mechanisms of bacterial factors have been established in the penetration of the BBB. The capsule can aid in bacterial transcellular crossing of the BBB, along with other attachment proteins83–85,104,118. Other mechanisms of crossing the BBB, including paracellular pathways or the “Trojan horse” mechanism of infected phagocytes, have been identified83–85,104. Meningitis can also occur through direct compromise of the BBB, and mechanisms include penetrating injuries, congenital defects, adjacent infections with erosion into the CNS, or neurosurgical procedures119–125.
Upon entry of bacterial pathogens into the CNS, they rapidly divide, as the CNS is devoid of complement, antibodies, and opsonic proteins85,86. The immune response is activated by Toll-like receptors and Nod-like receptors recognizing pathogen-associated molecular patterns126,127. These signaling pathways lead to the production of proinflammatory cytokines and mobilization of the immune response, leading to pleocytosis of white blood cells83–86,126. Bacterial cell wall material, enzymes, and toxins cause direct injury to neurons and indirect damage by increasing vascular permeability that causes edema and further injury83–86. Neuronal injury is also caused by toxic molecules released by the immune response, including reactive oxygen species, nitrous oxide, and peroxynitrite84–86. The release of proteases and excitatory amino acids by the immune response also contributes to neurotoxicity84–86.
Special considerations of young infant meningitis
Inoculation of bacteria into mucosal surfaces occurs prior to and during parturition with subsequent bacterial invasion causing early onset sepsis48. Late-onset sepsis is associated with a period of asymptomatic bacterial colonization and subsequent invasion3–5. Two sites of colonization likely contribute to cases of late-onset meningitis. The gastrointestinal tract serves as a frequent site of colonization for E. coli, GBS, and L. monocytogenes, and all of these pathogens have essential factors that allow for epithelial adherence and invasion48,85,128. GBS is readily transmitted from mother to neonate; 29–85% (mean rate of approximately 50%) of infants born to a GBS-positive mother become colonized48. The second potential site of colonization is the urinary tract, which may harbor asymptomatic bacteriuria129,130. Ascending infection can lead to seeding of the kidney, bacteremia, and then meningitis, as around 13.2% of febrile young infants will present with a urinary tract infection, and a smaller subset will have simultaneous evidence of bacteriuria, bacteremia, and meningitis44,131.
In premature infants, bacteremic events can be preceded by colonization of the gut by the causative pathogen132. In a prospective monitoring of stool samples, Carl et al. captured seven cases of sepsis in which the causative bacterial pathogen was also identified from the patient’s stool sample preceding the episode of bacteremia132. It is unclear whether similar events occur in term infants or within other body sites that contribute to invasion of these bacterial organisms. The effects of intrapartum antibiotics on the infant microbiome are also being elucidated, as the gastrointestinal microbiome of infants born to mothers who received intrapartum antibiotics is different from that of infants born to untreated mothers133–136. However, the consequences, if any, of these microbial communities for the risk of meningitis are unknown.
Recent analysis of the microbiome has shown dynamic colonization of the neonatal gut, and one potential factor in colonization is the population of bacteriophages137,138. In a longitudinal study of healthy twins, the neonatal bacterial microbiome gained bacterial diversity with increased age, and conversely bacteriophage diversity decreased with age137. This may suggest an essential relationship between bacteriophages and development of the gut bacterial microbiome, in which the bacteriophage population guides the diversity of the bacterial population. Further data are needed to determine whether the risk of invasive bacterial disease is increased by certain compositions of the microbiome and whether bacteriophage populations potentiate this risk139.
The relatively immature immune system of the neonate also contributes to the invasive risk of bacterial pathogens140–142. Defects in phagocytic cell function, chemotaxis, cytokine production, complement pathways, Toll-like receptor responses, and antibody production are further conducive to invasive disease140–142. These defects also include adaptive immunity in response to viral infections, including lymphocyte proliferation and antibody responses142–144. Though significant, these immune defects are transient, likely contributing to the decreased incidence of serious bacterial infections with increasing age.
Conclusions
The incidence of bacterial meningitis in children has been dramatically reduced and this is primarily because of immunization and intrapartum prophylaxis strategies. Nonetheless, E. coli, GBS, S. pneumoniae, and N. meningitidis remain important pathogens of meningitis. Recent advances in the analysis of the microbiome have expanded the understanding of the pathogenesis of meningitis. These new insights will provide new avenues of research and may stimulate the development of future treatments to prevent and treat meningitis.
Competing interests
The authors declare that they have no competing interests.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Simonsen KA, Anderson-Berry AL, Delair SF, et al.:
Early-onset neonatal sepsis.
Clin Microbiol Rev.
2014; 27(1): 21–47. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 2.
Le Doare K, Kampmann B:
Breast milk and Group B streptococcal infection: vector of transmission or vehicle for protection?
Vaccine.
2014; 32(26): 3128–32. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Berardi A, Cattelani C, Creti R, et al.:
Group B streptococcal infections in the newborn infant and the potential value of maternal vaccination.
Expert Rev Anti Infect Ther.
2015; 13(11): 1387–99. PubMed Abstract
| Publisher Full Text
- 4.
Dong Y, Speer CP:
Late-onset neonatal sepsis: recent developments.
Arch Dis Child Fetal Neonatal Ed.
2015; 100(3): F257–63. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Barichello T, Fagundes GD, Generoso JS, et al.:
Pathophysiology of neonatal acute bacterial meningitis.
J Med Microbiol.
2013; 62(Pt 12): 1781–9. PubMed Abstract
| Publisher Full Text
- 6.
Schlech WF 3rd, Ward JI, Band JD, et al.:
Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study.
JAMA.
1985; 253(12): 1749–54. PubMed Abstract
| Publisher Full Text
- 7.
Centers for Disease Control and Prevention: Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2014. 2014. Reference Source
- 8.
Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC):
Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP).
MMWR Recomm Rep.
2010; 59(RR-11): 1–18. PubMed Abstract
- 9.
Cox CM, Link-Gelles R:
Chapter 11: Pneumococcal. In Manual for the surveillance of vaccine-preventable diseases. (ed), Dept. of Health and Human Services Centers for Disease Control and Prevention, Atlanta, Ga. 2012. Reference Source
- 10.
Hsu HE, Shutt KA, Moore MR, et al.:
Effect of pneumococcal conjugate vaccine on pneumococcal meningitis.
N Engl J Med.
2009; 360(3): 244–56. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 11.
Brouwer MC, Tunkel AR, van de Beek D:
Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis.
Clin Microbiol Rev.
2010; 23(3): 467–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
McIntyre PB, O'Brien KL, Greenwood B, et al.:
Effect of vaccines on bacterial meningitis worldwide.
Lancet.
2012; 380(9854): 1703–11. PubMed Abstract
| Publisher Full Text
- 13.
Weinberger DM, Malley R, Lipsitch M:
Serotype replacement in disease after pneumococcal vaccination.
Lancet.
2011; 378(9807): 1962–73. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Moore MR, Link-Gelles R, Schaffner W, et al.:
Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance.
Lancet Infect Dis.
2015; 15(3): 301–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 15.
Levy C, Varon E, Picard C, et al.:
Trends of pneumococcal meningitis in children after introduction of the 13-valent pneumococcal conjugate vaccine in France.
Pediatr Infect Dis J.
2014; 33(12): 1216–21. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 16.
Andrews NJ, Waight PA, Burbidge P, et al.:
Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study.
Lancet Infect Dis.
2014; 14(9): 839–46. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 17.
Ben-Shimol S, Greenberg D, Givon-Lavi N, et al.:
Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: an active prospective nationwide surveillance.
Vaccine.
2014; 32(27): 3452–9. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 18.
Demczuk WH, Martin I, Griffith A, et al.:
Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012.
Can J Microbiol.
2013; 59(12): 778–88. PubMed Abstract
| Publisher Full Text
- 19.
Harboe ZB, Dalby T, Weinberger DM, et al.:
Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality.
Clin Infect Dis.
2014; 59(8): 1066–73. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 20.
Guevara M, Ezpeleta C, Gil-Setas A, et al.:
Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001–2013.
Vaccine.
2014; 32(22): 2553–62. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 21.
Olarte L, Barson WJ, Barson RM, et al.:
Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children.
Clin Infect Dis.
2015; 61(5): 767–75. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 22.
Cohen R, Biscardi S, Levy C:
The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014.
Hum Vaccin Immunother.
2016; 12(2): 277–84. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 23.
Thigpen MC, Whitney CG, Messonnier NE, et al.:
Bacterial meningitis in the United States, 1998–2007.
N Engl J Med.
2011; 364(21): 2016–25. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 24.
Pittman M:
Variation And Type Specificity In The Bacterial Species Hemophilus Influenzae.
J Exp Med.
1931; 53(4): 471–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Adams D, Fullerton K, Jajosky R, et al.:
Summary of Notifiable Infectious Diseases and Conditions - United States, 2013.
MMWR Morb Mortal Wkly Rep.
2015; 62(53): 1–122. PubMed Abstract
| Publisher Full Text
- 26.
Centers for Disease Control and Prevention:
Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Haemophilus influenza 2014. 2014. Reference Source
- 27.
Adams DA, Thomas KR, Jajosky RA, et al.:
Summary of Notifiable Infectious Diseases and Conditions - United States, 2014.
MMWR Morb Mortal Wkly Rep.
2016; 63(54): 1–152. PubMed Abstract
| Publisher Full Text
- 28.
Peltola H:
Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates.
Clin Microbiol Rev.
2000; 13(2): 302–17. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Rouphael NG, Stephens DS:
Neisseria meningitidis: biology, microbiology, and epidemiology.
Methods Mol Biol.
2012; 799: 1–20. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Cohn AC, MacNeil JR, Harrison LH, et al.:
Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease.
Clin Infect Dis.
2010; 50(2): 184–91. PubMed Abstract
| Publisher Full Text
- 31.
MacNeil JR, Bennett N, Farley MM, et al.:
Epidemiology of infant meningococcal disease in the United States, 2006–2012.
Pediatrics.
2015; 135(2): e305–11. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 32.
Sáfadi MA, McIntosh ED:
Epidemiology and prevention of meningococcal disease: a critical appraisal of vaccine policies.
Expert Rev Vaccines.
2011; 10(12): 1717–30. PubMed Abstract
| Publisher Full Text
- 33.
Borrow R, Alarcón P, Carlos J, et al.:
The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection.
Expert Rev Vaccines.
2016: 1–16. PubMed Abstract
| Publisher Full Text
- 34.
Scarborough M, Thwaites GE:
The diagnosis and management of acute bacterial meningitis in resource-poor settings.
Lancet Neurol.
2008; 7(7): 637–48. PubMed Abstract
| Publisher Full Text
- 35.
Cohn A, MacNeil J:
The Changing Epidemiology of Meningococcal Disease.
Infect Dis Clin North Am.
2015; 29(4): 667–77. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 36.
Collard J, Issaka B, Zaneidou M, et al.:
Epidemiological changes in meningococcal meningitis in Niger from 2008 to 2011 and the impact of vaccination.
BMC Infect Dis.
2013; 13: 576. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 37.
Gladstone IM, Ehrenkranz RA, Edberg SC, et al.:
A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience.
Pediatr Infect Dis J.
1990; 9(11): 819–25. PubMed Abstract
| Publisher Full Text
- 38.
Wiswell TE, Baumgart S, Gannon CM, et al.:
No lumbar puncture in the evaluation for early neonatal sepsis: will meningitis be missed?
Pediatrics.
1995; 95(6): 803–6. PubMed Abstract
- 39.
Camacho-Gonzalez A, Spearman PW, Stoll BJ:
Neonatal infectious diseases: evaluation of neonatal sepsis.
Pediatr Clin North Am.
2013; 60(6): 367–89. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 40.
Gaschignard J, Levy C, Romain O, et al.:
Neonatal Bacterial Meningitis: 444 Cases in 7 Years.
Pediatr Infect Dis J.
2011; 30(3): 212–7. PubMed Abstract
| Publisher Full Text
- 41.
Chang C, Chang WN, Huang LT, et al.:
Bacterial meningitis in infants: the epidemiology, clinical features, and prognostic factors.
Brain Dev.
2004; 26(3): 168–75. PubMed Abstract
| Publisher Full Text
- 42.
Edmond KM, Kortsalioudaki C, Scott S, et al.:
Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis.
Lancet.
2012; 379(9815): 547–56. PubMed Abstract
| Publisher Full Text
- 43.
Okike IO, Johnson AP, Henderson KL, et al.:
Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance.
Clin Infect Dis.
2014; 59(10): e150–7. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 44.
Greenhow TL, Hung YY, Herz AM, et al.:
The changing epidemiology of serious bacterial infections in young infants.
Pediatr Infect Dis J.
2014; 33(6): 595–9. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 45.
Byington CL, Rittichier KK, Bassett KE, et al.:
Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens.
Pediatrics.
2003; 111(5 Pt 1): 964–8. PubMed Abstract
| Publisher Full Text
- 46.
Sadow KB, Derr R, Teach SJ:
Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment.
Arch Pediatr Adolesc Med.
1999; 153(6): 611–4. PubMed Abstract
| Publisher Full Text
- 47.
Schrag SJ, Zywicki S, Farley MM, et al.:
Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis.
N Engl J Med.
2000; 342(1): 15–20. PubMed Abstract
| Publisher Full Text
- 48.
Edwards MS, Nizet V, Baker CJ:
Group B Streptococcal Infections. In Remington and Klein's infectious diseases of the fetus and newborn infant, 8th ed. Elsevier/Saunders, Philadelphia. 2016. Reference Source
- 49.
Prevention of perinatal group B streptococcal disease: a public health perspective. Centers for Disease Control and Prevention.
MMWR Recomm Rep.
1996; 45(RR-7): 1–24. PubMed Abstract
- 50.
ACOG committee opinion. Prevention of early-onset group B streptococcal disease in newborns. Number 173--June 1996. Committee on Obstetric Practice. American College of Obstetrics and Gynecologists.
Int J Gynaecol Obstet.
1996; 54(2): 197–205. PubMed Abstract
- 51.
Revised guidelines for prevention of early-onset group B streptococcal (GBS) infection. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn.
Pediatrics.
1997; 99(3): 489–96. PubMed Abstract
| Publisher Full Text
- 52.
Schrag S, Gorwitz R, Fultz-Butts K, et al.:
Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC.
MMWR Recomm Rep.
2002; 51(RR-11): 1–22. PubMed Abstract
- 53.
American College of Obstetricians and Gynecologists: ACOG Committee Opinion: number 279, December 2002. Prevention of early-onset group B streptococcal disease in newborns.
Obstet Gynecol.
2002; 100(6): 1405–12. PubMed Abstract
| Publisher Full Text
- 54.
Committee on Infectious Diseases, American Academy of Pediatrics, Kimberlin DW BMT, , et al.:
Group B Streptococcal infections in Red book 2003 : report of the Committee on Infectious Diseases. 26. ed. American Academy of Pediatrics; BMJ, Washington, D.C. London. 2003; 927.Reference Source
- 55.
Verani JR, McGee L, Schrag SJ, et al.:
Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010.
MMWR Recomm Rep.
2010; 59(RR-10): 1–36. PubMed Abstract
- 56.
de Cueto M, Sanchez MJ, Sampedro A, et al.:
Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus.
Obstet Gynecol.
1998; 91(1): 112–4. PubMed Abstract
| Publisher Full Text
- 57.
Turrentine MA, Greisinger AJ, Brown KS, et al.:
Duration of intrapartum antibiotics for group B streptococcus on the diagnosis of clinical neonatal sepsis.
Infect Dis Obstet Gynecol.
2013; 2013: 525878. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 58.
Phares CR, Lynfield R, Farley MM, et al.:
Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005.
JAMA.
2008; 299(17): 2056–65. PubMed Abstract
| Publisher Full Text
- 59.
Jordan HT, Farley MM, Craig A, et al.:
Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis.
Pediatr Infect Dis J.
2008; 27(12): 1057–64. PubMed Abstract
| Publisher Full Text
- 60.
Basmaci R, Bonacorsi S, Bidet P, et al.:
Escherichia Coli Meningitis Features in 325 Children From 2001 to 2013 in France.
Clin Infect Dis.
2015; 61(5): 779–86. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 61.
Baltimore RS, Huie SM, Meek JI, et al.:
Early-onset neonatal sepsis in the era of group B streptococcal prevention.
Pediatrics.
2001; 108(5): 1094–8. PubMed Abstract
| Publisher Full Text
- 62.
Edwards RK, Jamie WE, Sterner D, et al.:
Intrapartum antibiotic prophylaxis and early-onset neonatal sepsis patterns.
Infect Dis Obstet Gynecol.
2003; 11(4): 221–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 63.
Daley AJ, Isaacs D; Australasian Study Group for Neonatal Infections:
Ten-year study on the effect of intrapartum antibiotic prophylaxis on early onset group B streptococcal and Escherichia coli neonatal sepsis in Australasia.
Pediatr Infect Dis J.
2004; 23(7): 630–4. PubMed Abstract
| Publisher Full Text
- 64.
Bauserman MS, Laughon MM, Hornik CP, et al.:
Group B Streptococcus and Escherichia coli infections in the intensive care nursery in the era of intrapartum antibiotic prophylaxis.
Pediatr Infect Dis J.
2013; 32(3): 208–12. PubMed Abstract
| Free Full Text
- 65.
Bizzarro MJ, Dembry L, Baltimore RS, et al.:
Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis.
Pediatrics.
2008; 121(4): 689–96. PubMed Abstract
| Publisher Full Text
- 66.
Stoll BJ, Hansen N, Fanaroff AA, et al.:
Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants.
N Engl J Med.
2002; 347(4): 240–7. PubMed Abstract
| Publisher Full Text
- 67.
Schrag SJ, Hadler JL, Arnold KE, et al.:
Risk factors for invasive, early-onset Escherichia coli infections in the era of widespread intrapartum antibiotic use.
Pediatrics.
2006; 118(2): 570–6. PubMed Abstract
| Publisher Full Text
- 68.
Moore MR, Schrag SJ, Schuchat A:
Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis.
Lancet Infect Dis.
2003; 3(4): 201–13. PubMed Abstract
| Publisher Full Text
- 69.
Puopolo KM, Eichenwald EC:
No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years.
Pediatrics.
2010; 125(5): e1031–8. PubMed Abstract
| Publisher Full Text
- 70.
Moore AM, Ahmadi S, Patel S, et al.:
Gut resistome development in healthy twin pairs in the first year of life.
Microbiome.
2015; 3: 27. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 71.
Gurnee EA, Ndao IM, Johnson JR, et al.:
Gut Colonization of Healthy Children and Their Mothers With Pathogenic Ciprofloxacin-Resistant Escherichia coli.
J Infect Dis.
2015; 212(12): 1862–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 72.
Gibson MK, Wang B, Ahmadi S, et al.:
Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome.
Nat Microbiol.
2016; 1: 16024. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 73.
Gibson MK, Crofts TS, Dantas G:
Antibiotics and the developing infant gut microbiota and resistome.
Curr Opin Microbiol.
2015; 27: 51–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 74.
Gasparrini AJ, Crofts TS, Gibson MK, et al.:
Antibiotic perturbation of the preterm infant gut microbiome and resistome.
Gut Microbes.
2016; 7(5): 443–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 75.
Centers for Disease Control and Prevention: National Listeria surveillance annual summary, 2014. 2015. Reference Source
- 76.
Tappero JW, Schuchat A, Deaver KA, et al.:
Reduction in the incidence of human listeriosis in the United States. Effectiveness of prevention efforts? The Listeriosis Study Group.
JAMA.
1995; 273(14): 1118–22. PubMed Abstract
| Publisher Full Text
- 77.
Lamont RF, Sobel J, Mazaki-Tovi S, et al.:
Listeriosis in human pregnancy: a systematic review.
J Perinat Med.
2011; 39(3): 227–36. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 78.
Jackson KA, Iwamoto M, Swerdlow D:
Pregnancy-associated listeriosis.
Epidemiol Infect.
2010; 138(10): 1503–9. PubMed Abstract
| Publisher Full Text
- 79.
Shank FR, Elliot EL, Wachsmuth I, et al.:
US position on Listeria monocytogenes in foods.
Food Control.
1996; 7(4–5): 229–34. Publisher Full Text
- 80.
Silk BJ, Date KA, Jackson KA, et al.:
Invasive listeriosis in the Foodborne Diseases Active Surveillance Network (FoodNet), 2004–2009: further targeted prevention needed for higher-risk groups.
Clin Infect Dis.
2012; 54(Suppl 5): S396–404. PubMed Abstract
| Publisher Full Text
- 81.
Ventura SJ, Abma JC, Mosher WD, et al.:
Estimated pregnancy rates by outcome for the United States, 1990–2004.
Natl Vital Stat Rep.
2008; 56(15): 1–25, 28. PubMed Abstract
- 82.
Lee B, Newland JG, Jhaveri R:
Reductions in neonatal listeriosis: "Collateral benefit" of Group B streptococcal prophylaxis?
J Infect.
2016; 72(3): 317–23. PubMed Abstract
| Publisher Full Text
- 83.
Dando SJ, Mackay-Sim A, Norton R, et al.:
Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion.
Clin Microbiol Rev.
2014; 27(4): 691–726. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 84.
Kim KS:
Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury.
Nat Rev Neurosci.
2003; 4(5): 376–85. PubMed Abstract
| Publisher Full Text
- 85.
Doran KS, Fulde M, Gratz N, et al.:
Host-pathogen interactions in bacterial meningitis.
Acta Neuropathol.
2016; 131(2): 185–209. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 86.
Liechti FD, Grandgirard D, Leib SL:
Bacterial meningitis: insights into pathogenesis and evaluation of new treatment options: a perspective from experimental studies.
Future Microbiol.
2015; 10(7): 1195–213. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 87.
Robinson J:
Colonization and infection of the respiratory tract: What do we know?
Paediatr Child Health.
2004; 9(1): 21–4. PubMed Abstract
| Free Full Text
- 88.
Bosch AA, Biesbroek G, Trzcinski K, et al.:
Viral and bacterial interactions in the upper respiratory tract.
PLoS Pathog.
2013; 9(1): e1003057. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 89.
Pericone CD, Overweg K, Hermans PW, et al.:
Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract.
Infect Immun.
2000; 68(7): 3990–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 90.
Margolis E, Yates A, Levin BR:
The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response.
BMC Microbiol.
2010; 10: 59. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 91.
Lysenko ES, Ratner AJ, Nelson AL, et al.:
The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces.
PLoS Pathog.
2005; 1(1): e1. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 92.
Devine VT, Jefferies JM, Clarke SC, et al.:
Nasopharyngeal Bacterial Carriage in the Conjugate Vaccine Era with a Focus on Pneumococci.
J Immunol Res.
2015; 2015: 394368. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 93.
Ngo CC, Massa HM, Thornton RB, et al.:
Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review.
PLoS One.
2016; 11(3): e0150949. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 94.
Dunne EM, Smith-Vaughan HC, Robins-Browne RM, et al.:
Nasopharyngeal microbial interactions in the era of pneumococcal conjugate vaccination.
Vaccine.
2013; 31(19): 2333–42. PubMed Abstract
| Publisher Full Text
- 95.
de Steenhuijsen Piters WA, Bogaert D:
Unraveling the Molecular Mechanisms Underlying the Nasopharyngeal Bacterial Community Structure.
MBio.
2016; 7(1): e00009–16. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 96.
Ampofo K, Bender J, Sheng X, et al.:
Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection.
Pediatrics.
2008; 122(2): 229–37. PubMed Abstract
| Publisher Full Text
- 97.
Sajjan U, Wang Q, Zhao Y, et al.:
Rhinovirus disrupts the barrier function of polarized airway epithelial cells.
Am J Respir Crit Care Med.
2008; 178(12): 1271–81. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 98.
Suzuki K, Bakaletz LO:
Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media.
Infect Immun.
1994; 62(5): 1710–8. PubMed Abstract
| Free Full Text
- 99.
Mueller JE, Gessner BD:
A hypothetical explanatory model for meningococcal meningitis in the African meningitis belt.
Int J Infect Dis.
2010; 14(7): e553–9. PubMed Abstract
| Publisher Full Text
- 100.
Bharti N, Broutin H, Grais RF, et al.:
Spatial dynamics of meningococcal meningitis in Niger: observed patterns in comparison with measles.
Epidemiol Infect.
2012; 140(8): 1356–65. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 101.
Cohen AL, McMorrow M, Walaza S, et al.:
Potential Impact of Co-Infections and Co-Morbidities Prevalent in Africa on Influenza Severity and Frequency: A Systematic Review.
PLoS One.
2015; 10(6): e0128580. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 102.
Jansen AG, Sanders EA, VAN DER Ende A, et al.:
Invasive pneumococcal and meningococcal disease: association with influenza virus and respiratory syncytial virus activity?
Epidemiol Infect.
2008; 136(11): 1448–54. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 103.
Talbot TR, Poehling KA, Hartert TV, et al.:
Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation.
Am J Med.
2005; 118(3): 285–91. PubMed Abstract
| Publisher Full Text
- 104.
Kim KS:
Mechanisms of microbial traversal of the blood-brain barrier.
Nat Rev Microbiol.
2008; 6(8): 625–34. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 105.
Sutherland TC, Quattroni P, Exley RM, et al.:
Transcellular passage of Neisseria meningitidis across a polarized respiratory epithelium.
Infect Immun.
2010; 78(9): 3832–47. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 106.
Spinosa MR, Progida C, Talà A, et al.:
The Neisseria meningitidis capsule is important for intracellular survival in human cells.
Infect Immun.
2007; 75(7): 3594–603. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 107.
Nikulin J, Panzner U, Frosch M, et al.:
Intracellular survival and replication of Neisseria meningitidis in human brain microvascular endothelial cells.
Int J Med Microbiol.
2006; 296(8): 553–8. PubMed Abstract
| Publisher Full Text
- 108.
Magee AD, Yother J:
Requirement for capsule in colonization by Streptococcus pneumoniae.
Infect Immun.
2001; 69(6): 3755–61. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 109.
Nelson AL, Roche AM, Gould JM, et al.:
Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance.
Infect Immun.
2007; 75(1): 83–90. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 110.
Weiser JN:
The pneumococcus: why a commensal misbehaves.
J Mol Med (Berl).
2010; 88(2): 97–102. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 111.
Turk DC:
The pathogenicity of Haemophilus influenzae.
J Med Microbiol.
1984; 18(1): 1–16. PubMed Abstract
| Publisher Full Text
- 112.
Stephens DS, Greenwood B, Brandtzaeg P:
Epidemic meningitis, meningococcaemia, and Neisseria meningitidis.
Lancet.
2007; 369(9580): 2196–210. PubMed Abstract
| Publisher Full Text
- 113.
Overturf GD:
Indications for the immunological evaluation of patients with meningitis.
Clin Infect Dis.
2003; 36(2): 189–94. PubMed Abstract
| Publisher Full Text
- 114.
Kim KS, Itabashi H, Gemski P, et al.:
The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat.
J Clin Invest.
1992; 90(3): 897–905. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 115.
Dietzman DE, Fischer GW, Schoenknecht FD:
Neonatal Escherichia coli septicemia--bacterial counts in blood.
J Pediatr.
1974; 85(1): 128–30. PubMed Abstract
| Publisher Full Text
- 116.
Sullivan TD, LaScolea LJ Jr, Neter E:
Relationship between the magnitude of bacteremia in children and the clinical disease.
Pediatrics.
1982; 69(6): 699–702. PubMed Abstract
- 117.
Bell LM, Alpert G, Campos JM, et al.:
Routine quantitative blood cultures in children with Haemophilus influenzae or Streptococcus pneumoniae bacteremia.
Pediatrics.
1985; 76(6): 901–4. PubMed Abstract
- 118.
Fuchs E, Untucht C, Rohde M:
Capsule Contributes to Transmigration of Streptococcus pneumoniae Serotype 7F Meningitis Isolates through Complex Blood Brain Barrier Models.
J Bacteriol Parasitol.
2014; 03. Publisher Full Text
- 119.
Baltas I, Tsoulfa S, Sakellariou P, et al.:
Posttraumatic meningitis: bacteriology, hydrocephalus, and outcome.
Neurosurgery.
1994; 35(3): 422–6; discussion 426-7. PubMed Abstract
| Publisher Full Text
- 120.
Ginsberg L, Kidd D:
Chronic and recurrent meningitis.
Pract Neurol.
2008; 8(6): 348–61. PubMed Abstract
| Publisher Full Text
- 121.
van den Aardweg MT, Rovers MM, de Ru JA, et al.:
A systematic review of diagnostic criteria for acute mastoiditis in children.
Otol Neurotol.
2008; 29(6): 751–7. PubMed Abstract
| Publisher Full Text
- 122.
Samuel J, Fernandes CM, Steinberg JL:
Intracranial otogenic complications: a persisting problem.
Laryngoscope.
1986; 96(3): 272–8. PubMed Abstract
| Publisher Full Text
- 123.
Younis RT, Anand VK, Childress C:
Sinusitis complicated by meningitis: current management.
Laryngoscope.
2001; 111(8): 1338–42. PubMed Abstract
| Publisher Full Text
- 124.
Simon TD, Butler J, Whitlock KB, et al.:
Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study.
J Pediatr.
2014; 164(6): 1462–8.e2. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 125.
Reefhuis J, Honein MA, Whitney CG, et al.:
Risk of bacterial meningitis in children with cochlear implants.
N Engl J Med.
2003; 349(5): 435–45. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 126.
Brouwer MC, de Gans J, Heckenberg SG, et al.:
Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis.
Lancet Infect Dis.
2009; 9(1): 31–44. PubMed Abstract
| Publisher Full Text
- 127.
Kanneganti TD, Lamkanfi M, Núñez G:
Intracellular NOD-like receptors in host defense and disease.
Immunity.
2007; 27(4): 549–59. PubMed Abstract
| Publisher Full Text
- 128.
Vázquez-Boland JA, Kuhn M, Berche P, et al.:
Listeria pathogenesis and molecular virulence determinants.
Clin Microbiol Rev.
2001; 14(3): 584–640. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 129.
Whiteside SA, Razvi H, Dave S, et al.:
The microbiome of the urinary tract--a role beyond infection.
Nat Rev Urol.
2015; 12(2): 81–90. PubMed Abstract
| Publisher Full Text
- 130.
Wettergren B, Jodal U:
Spontaneous clearance of asymptomatic bacteriuria in infants.
Acta Paediatr Scand.
1990; 79(3): 300–4. PubMed Abstract
| Publisher Full Text
- 131.
Tebruegge M, Pantazidou A, Curtis N:
Question 1. How common is co-existing meningitis in infants with urinary tract infection?
Arch Dis Child.
2011; 96(6): 602–6. PubMed Abstract
| Publisher Full Text
- 132.
Carl MA, Ndao IM, Springman AC, et al.:
Sepsis from the gut: the enteric habitat of bacteria that cause late-onset neonatal bloodstream infections.
Clin Infect Dis.
2014; 58(9): 1211–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 133.
Aloisio I, Mazzola G, Corvaglia LT, et al.:
Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains.
Appl Microbiol Biotechnol.
2014; 98(13): 6051–60. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 134.
Keski-Nisula L, Kyynäräinen H, Kärkkäinen U, et al.:
Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth.
Acta Paediatr.
2013; 102(5): 480–5. PubMed Abstract
| Publisher Full Text
- 135.
Mazzola G, Murphy K, Ross RP, et al.:
Early Gut Microbiota Perturbations Following Intrapartum Antibiotic Prophylaxis to Prevent Group B Streptococcal Disease.
PLoS One.
2016; 11(6): e0157527. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 136.
Azad MB, Konya T, Persaud RR, et al.:
Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study.
BJOG.
2016; 123(6): 983–93. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 137.
Lim ES, Zhou Y, Zhao G, et al.:
Early life dynamics of the human gut virome and bacterial microbiome in infants.
Nat Med.
2015; 21(10): 1228–34. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 138.
La Rosa PS, Warner BB, Zhou Y, et al.:
Patterned progression of bacterial populations in the premature infant gut.
Proc Natl Acad Sci U S A.
2014; 111(34): 12522–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 139.
Norman JM, Handley SA, Virgin HW:
Kingdom-agnostic metagenomics and the importance of complete characterization of enteric microbial communities.
Gastroenterology.
2014; 146(6): 1459–69. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 140.
Maródi L:
Neonatal innate immunity to infectious agents.
Infect Immun.
2006; 74(4): 1999–2006. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 141.
Levy O:
Innate immunity of the newborn: basic mechanisms and clinical correlates.
Nat Rev Immunol.
2007; 7(5): 379–90. PubMed Abstract
| Publisher Full Text
- 142.
Ygberg S, Nilsson A:
The developing immune system - from foetus to toddler.
Acta Paediatr.
2012; 101(2): 120–7. PubMed Abstract
| Publisher Full Text
- 143.
Burchett SK, Corey L, Mohan KM, et al.:
Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection.
J Infect Dis.
1992; 165(5): 813–8. PubMed Abstract
| Publisher Full Text
- 144.
Sullender WM, Miller JL, Yasukawa LL, et al.:
Humoral and cell-mediated immunity in neonates with herpes simplex virus infection.
J Infect Dis.
1987; 155(1): 28–37. PubMed Abstract
| Publisher Full Text



Comments on this article Comments (0)