Keywords
food emerging pathogens, Saccharomyces cerevisiae, blood brain barrier, virulence,
food emerging pathogens, Saccharomyces cerevisiae, blood brain barrier, virulence,
In this new version, we have considered HUVEC cells as a model of endothelial cells instead of a blood brain barrier.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Saccharomyces cerevisiae is generally considered safe, and is involved in the production of a variety of foods and dietary supplements. Several types of food and beverage still contain viable yeast cells1–5. However, in the last years human infections with Saccharomyces cerevisiae have increased6–8. Consequently, S. cerevisiae is considered an emerging pathogen9–11. Different parts of the body can be affected in immunocompromised12–15 and healthy patients16–18. The potential virulence of this yeast has been analysed with different methods in vitro19–22 and in vivo23–27, for example by measuring epithelial barrier traversal28. These reports have suggested that certain strains can cause disease and death in murine models. However, the bio-therapeutic agent Ultralevure (S. cerevisiae var. boulardii) and other supplements are consumed in high doses, ranging from 107 to 1010 live yeast cells per day and for long periods.
The study of yeast virulence includes studying their behaviour when they encounter endothelial barriers. Opportunistic pathogenic yeasts such as C. glabrata and C. albicans are able to pass the intestinal barrier29,30 and generate systemic infections31–33. Also, C. albicans can cross the blood-brain barrier (BBB) to reach the brain34,35. Regarding S. cerevisiae, infections after oral ingestion16 or digestive translocation12,14,36 show that it can reach brain in murine models25. However, few studies have investigated the behaviour of S. cerevisiae when they reach endothelial barriers28.
The yeast strains are described in Table 1. Strains were propagated in YPD media (1% glucose, 1% BactoPeptone, 0.5% yeast extract) for 24 h at 30°C.
| Strain | Species | Source |
|---|---|---|
| W303 | S. cerevisiae | From our collection |
| 102 | S. cerevisiae | Vall d’Hebron Hospital (Barcelona, Spain)19 |
| 60 | S. cerevisiae | Vall d’Hebron Hospital (Barcelona, Spain)19 |
| Cb | S. cerevisiae | Vall d’Hebron Hospital (Barcelona, Spain)19 |
| Co | C. glabrata | Vall d’Hebron Hospital (Barcelona, Spain) |
| C2 | C. glabrata | Provided by B. Hube (Friedrich Schiller University; Jena, Germany) |
| C4 | C. glabrata | Provided by B. Hube (Friedrich Schiller University; Jena, Germany) |
| C5 | C. glabrata | Provided by B. Hube (Friedrich Schiller University; Jena, Germany) |
| CA-1 | C. albicans | Statens Serum Institute (Copenhagen, Denmark) |
| SC5314 | C. albicans | Provided by A. Yañez22 (Universitat de Valencia, Spain) |
| ATCC26555 | C. albicans | Provided by A. Yañez22 (Universitat de Valencia, Spain) |
| CBS562 | C. albicans | From our collection |
Human umbilical endothelial cells (HUVECs) (Clonetics®) were grown in minimum essential medium (Earle’s salt, 25 mM HEPES and GlutaMAX™, Invitrogen) supplemented with 10% foetal bovine serum (FBS, Cambrex Bio Science), 1% nonessential amino acids (Invitrogen) and 50 μg mL–1 gentamicin (Invitrogen). The cells were grown in 150 cm2 culture flasks (TPP) at 37°C in a humidified atmosphere of 5% CO2 and 95% air until a confluence. Culture medium was changed every second day.
HUVEC cells (1×105 cells/cm2) were seeded on Transwell® filter inserts (8 μm, Corning Incorporated) in 24-well plates (Corning Incorporated). A volume of 200 μL cell growth medium was added to the apical compartment and 1250 μL to the basolateral compartment. The TEER was measured using the Millicell-ERS Electrical Resistance System (Millipore). The net value of the TEER (Ωcm2) was corrected for background resistance by subtracting the contribution of the cell-free filter and the medium (110 Ωcm2). The TEER was measured before the addition of yeasts.
1 μg/mL of fluorescein (Sigma) was added to the media in the apical compartment of the transwell, with or without established HUVEC monolayers, and fluorescence was measured over time in the media of the apical and basolateral compartment. The apparent permeability, Papp, was defined as (Hilgers et al., 1990):
Papp = (ΔAR/Δt))/CD,0 (1)
(ΔAR/Δt) is the rate of drug appearance in the receiver side, S is the surface area of the Transwell (0.33 cm2 for Transwell® inserts (8 μm pore size, Corning) of 6.5-mm insert diameter), and CD,0 is the initial drug concentration in the donor side at time = 0. Values are expressed in cm/s.
HUVEC cells were seeded on Transwell® filter as described above. Yeasts grown overnight at 30°C in YPD were resuspended (106 cells mL–1) in the apical compartment and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After 12 h, the basolateral compartment medium was replaced. Colony forming units were counted in YPD plate triplicates after two days. Control wells used to evaluate yeast growth showed no significant growth after 12 h. Negative control HUVEC Transwells without adding cells were performed to control TEER stability across the experiment.
To establish an in vitro human endothelial barrier, we used HUVEC monolayers, a methodology that has been widely used37,38. Monolayers were formed in transwells and two different methods were used to determine the robustness, consistency and integrity of the barrier. First, we studied the TEER, indicative of physical separation. After seeding the HUVECs, TEER was measured and we observed increased values over time that were overcoming 450 Ωcm2, which correlates with the establishment of a monolayer barrier. Second, we studied the monolayer permeability. The value obtained was 1.82±0.13 (10-6 cm/s) on average, which indicates an integral barrier with low permeability39.
To determine whether S. cerevisiae is able to cross the human endothelial barrier, we used an in vitro model of the endothelium with HUVECs40. The number of cells in the basolateral compartment was measured 12 hours after addition of S. cerevisiae, C. albicans and C. glabrata strains to the apical compartment (Figure 1). The results showed that all yeast strains were able to cross the endothelial barrier. While elevated number of cells from C. glabrata and C. albicans strains were able to cross the endothelial barrier, S. cerevisiae values were low. Furthermore, while the S. cerevisiae control strain W303 showed the lowest levels of yeast transcytosis, the other opportunistic pathogenic strains presented higher levels.
To compare the different species, the average level of cell transcytosis for all strains of each species was calculated (Figure 2). After 12 h, Candida species showed a high number of cells in the basolateral chamber (4.9–5.7 Log10 units). On the contrary, we observed that S. cerevisiae showed significantly lower levels (1.0–3.3 Log10 units) than the Candida species.
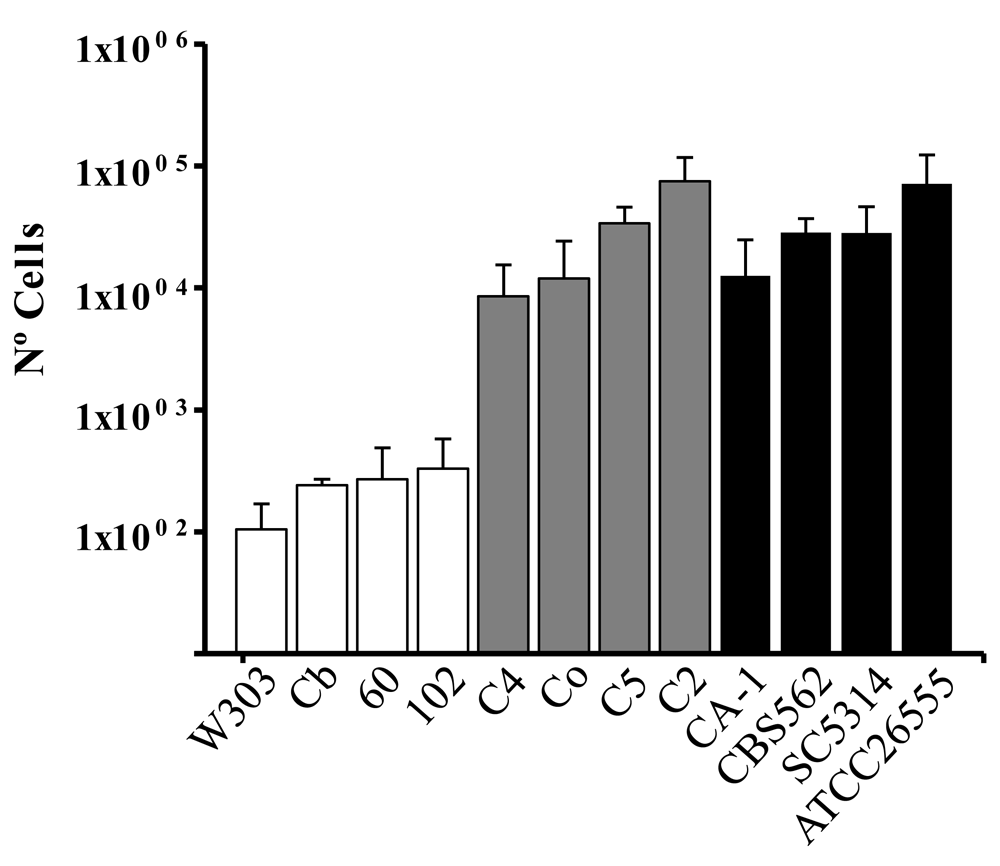
To perform this assay we established HUVEC monolayers in Transwell® filter inserts in 24 well plates. 24 hours after apical addition of various strains of S. cerevisiae, C. albicans and C. glabrata, yeast cells from the basolateral compartment were incubated on YPD plates and colonies were counted after one day of growth. Values were obtained after plating several dilutions of the basolateral compartment media. Average of three experiments and standard deviation is shown. To determine statistically significant data, Student t-tests were performed in Excel with 0.05 as the p-value.
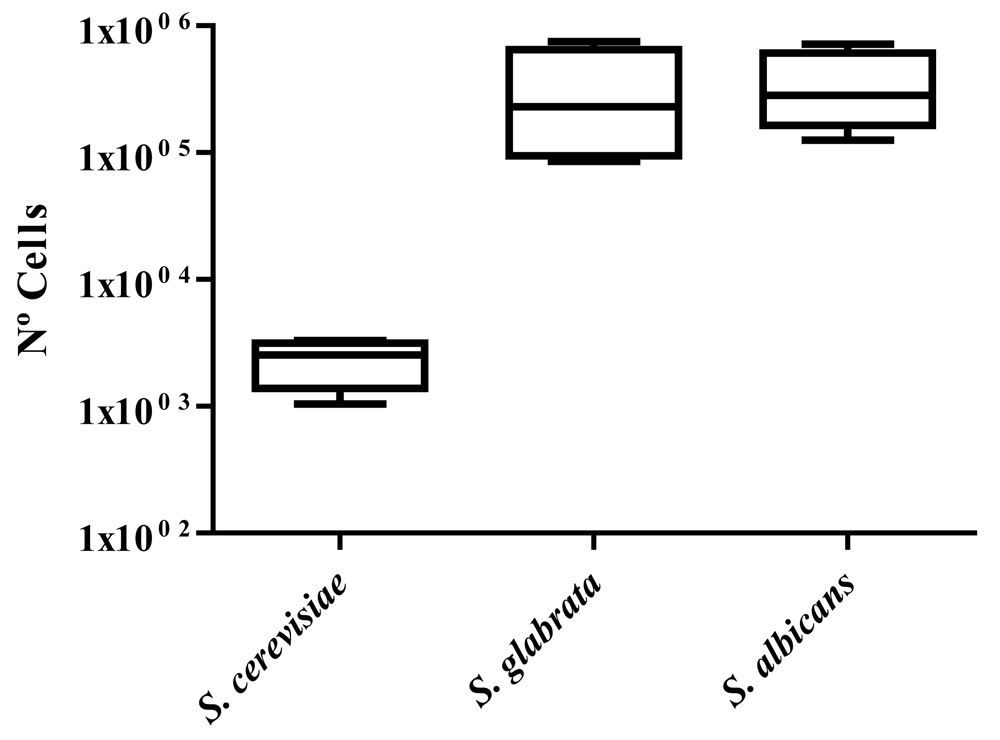
A model for traversal across the e in vitro has been used to study behaviour and pathogenicity mechanisms of yeast strains such as C. albicans34,35. Here, we have shown that S. cerevisiae strains are able to cross the endothelial barrier. This data is in accordance with previous studies, where S. cerevisiae cells were observed in the brain after systemic infections in murine models25. When comparing to other well-known yeast pathogens such as C. glabrata and C. albicans, none of the S. cerevisiae strains were able to cross the endothelial barrier at high levels. Despite S. cerevisiae pathogenicity levels being lower than other opportunistic yeasts, we recommend the potential risk of new S. cerevisiae strains to be evaluated before using them in food production.
Dataset 1: Raw data of permeability measurements and cell counts for endothelium traversal. DOI, 10.5256/f1000research.11782.d17755441
This work was supported by grants AGL2012-39937-C02-01 and AGL2015-67504-C3-1-R from the Spanish Government and ERDF (European Regional Development Fund) and by grant PROMETEO (project PROMETEOII/2014/042) from Generalitat Valenciana to AQ.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fungal pathogenesis, membrane trafficking
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fungal pathogenesis
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fungal pathogenesis, food microbiology, environmental microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 12 Sep 17 |
read | |
|
Version 1 20 Jun 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)