Keywords
Heart transplantation, organ allocation, transplant rejection, sensitization, crossmatch
Heart transplantation, organ allocation, transplant rejection, sensitization, crossmatch
Despite advances in medical and electrical therapies for heart failure, morbidity and mortality remain high and patients often progress to end-stage heart failure. Over the last five decades, heart transplantation is considered a standard therapy for select patients with end-stage heart failure. In the present era, one-year survival after heart transplantation is almost 90% and has a conditional half-life of 13 years1, which is superior to that of end-stage heart failure.
The number of patients with heart failure requiring advanced therapies is growing, while the number of donor organs remains a constant and limiting factor2. Heart transplant (HTx) candidates of the current era are also more complex. Increasing numbers are aged 65 years or more3, have mechanical circulatory support3, and have higher levels of antibodies to human leukocyte antigens (HLA), i.e. “sensitization”4. Due to all these issues, these HTx candidates of the modern era are at increased risk for poor outcomes, including primary graft dysfunction and antibody-mediated rejection1,2,5. The latest developments might be able to counter existing problems: 1) attempts to expand the pool of potential organ donors; 2) changes in the heart transplant donor allocation policy to allow for more equitable organ distribution; and 3) management of sensitized HTx candidates. Such advances could produce an increase in and a fairer allocation of donor organs and improved quality of life and survival for HTx recipients.
Currently, fewer than 50% of potential donors in the United States become actual organ donors6. Initiatives have been taken to increase the utilization rate7. Using less-than pristine donor hearts, so-called extended criteria donors, is one option to expand the donor pool. These hearts may be used for higher-risk recipients, such as those who are older, over age 65, with diabetes, renal dysfunction, or peripheral vascular disease. Considerable evidence shows that extended criteria donor hearts that may result in favorable post-HTx survival continue to be underutilized. In a retrospective review in California from 2001 to 2008 looking at 1872 possible organ donors found predictors of non-use to be age >50 years, female gender, fatal cerebrovascular accident, hypertension, diabetes mellitus, an elevated troponin, left ventricular dysfunction (ejection fraction <50%), left ventricular hypertrophy, and regional wall motion abnormalities. However, when such so-called extended criteria donor hearts are used for transplantation, outcomes are generally comparable8–11.
The donor pool could be expanded by limiting cold ischemic time. An ex vivo perfusion platform would allow the donor heart to be kept in a warm, beating state while being transported until the time of implantation. Small registry studies have demonstrated its safety12. In a randomized trial examining the safety of an ex vivo platform, donor hearts managed with either the Organ Care System or standard cold storage were randomized to 130 patients. There was no difference in 30-day patient survival rates, 30-day graft survival rates, or serious adverse events13.
The ex vivo perfusion platform may be particularly beneficial when used in conjunction with extended criteria donor hearts. Such donors, including those of older age, with left ventricular hypertrophy or moderately reduced ejection fraction, are more susceptible to injury with long ischemic time. Further study is needed to demonstrate the specific benefit of an ex-vivo perfusion platform in this population.
The allocation of donor hearts in the United States appears to be fair, as those patients who are sickest and who have been waiting the longest are the first to be considered in the event of a donor heart becoming available (Table 1). However, changes in the HTx landscape have motivated efforts to improve the current system14,15: there is an imbalance between candidates awaiting transplantation and available donors, the sickest patients have unacceptably high mortality, and advances in mechanical circulatory support have decreased mortality in these transplant candidates.
These alterations have uncovered two significant difficulties with the present status criteria. First, the system offers inadequate resolution. Status 1A includes the following groups with equal urgency: patients who receive extracorporeal membrane oxygenation (ECMO) and continuous intravenous inotropic support and hemodynamic monitoring. However, potential HTx recipients on ECMO support have increased mortality compared with candidates on inotropic support with continuous hemodynamic monitoring. Thus, these two groups of patients should not have the same priority, though they do under the current three-tiered system. The current system also makes no allowance for tenuous transplant candidates who do not qualify for Status 1A listing, including candidates with complex congenital heart disease, restrictive or infiltrative cardiomyopathies, or refractory ventricular tachycardia14,15.
There are seven statuses in the new allocation policy (Table 2)16. Proposed statuses 1–3 are generally defined by current Status 1A criteria, proposed Status 4 is generally defined by current Status 1B, and proposed Status 5 and 6 are covered by current Status 2 criteria. The most significant alteration is the classification of patients within the current 1A status into three groups of decreasing acuity. In addition, the suggested policy tackles potentially underserved groups, such as adults with congenital heart disease, restrictive/hypertrophic cardiomyopathy, and re-transplantation in a separate tier above current Status 2 patients.
BiVAD, biventricular assist device; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulator support; RVAD, right ventricular assist device; TAH, total artificial heart; VA, venoarterial.
This table was adapted with permission from Meyer DM, Rogers JG, Edwards LB, et al., The future direction of the adult heart allocation system in the United States. Am J Transplant 2015;15:44-54 and https://optn.transplant.hrsa.gov/media/2028/thoracic_policynotice_201612.pdf (accessed May 24, 2017).
The novel allocation system deals with geographical inequality in organ distribution (Figure 1) by broader sharing for the highest tier patients. The two highest-acuity groups will draw organs from a 500-mile radius in the first round of organ allocation instead of using a stepped approach.
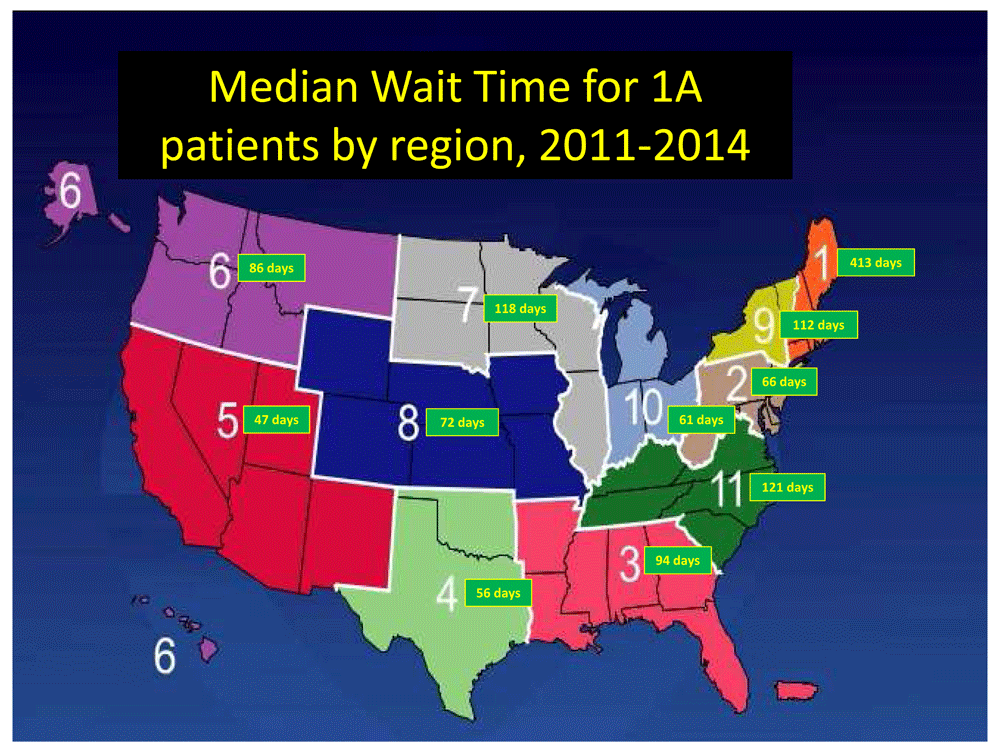
There is significant variation by region, as defined by the United Network for Organ Sharing (UNOS), in the median wait time for status 1A patients. Data from https://optn.transplant.hrsa.gov/data/view-data-reports/regional-data/.
This system was approved by the United Network of Organ Sharing Board and Organ Procurement and Transplant Network in December 2016. It will go into effect in the last quarter of 2018. No system can be perfect, but these efforts allow for more equitable distribution of this scarce resource and achieve the primary goal, whereby the most critically ill patients can receive transplantation before their window of viability closes.
Despite the fact that endomyocardial biopsy is usually a simple procedure, the morbidity associated has motivated development of other means to diagnose rejection. The gene expression profile test (AlloMap®, CareDx Inc, San Francisco, CA), an 11-gene expression signature from peripheral blood mononuclear cells, has a high negative predictive value for cellular rejection17 and is noninvasive. In randomized trials, gene expression profile was non-inferior to an endomyocardial biopsy in the diagnosis of cellular rejection18 and also useful early post-transplant19. One role of the gene expression profile is to screen patients at low risk for cellular rejection at pre-determined intervals with biopsies performed only in cases in which the gene expression profile score is abnormal. However, patients with risk factors for antibody-mediated rejection cannot be tested with gene expression profile screening as the technique can only be used to assess cellular rejection.
One more novel technique that can be used for noninvasive diagnosis of rejection uses cell-free DNA. Cell-free donor-derived DNA can be detected in the blood and urine of organ transplant recipients20,21 This cell-free DNA may be a potential marker for noninvasive diagnosis of graft injury, as cellular and antibody-mediated rejection events are associated with increased levels of cell-free donor-derived DNA22.
As mentioned above, the Allomap relies on peripheral blood gene expression profiling to refine diagnostic accuracy and does not detect antibody-mediated rejection (AMR). A recent study, however, studied mRNA extracted from endomyocardial biopsy samples and hybridized to a microarray system. Differences in AMR-selective gene expression classified AMR were linked with disease activity and ISHLT AMR grade23. This advance has the potential to refine diagnostic accuracy and may in the future provide insight into the management of AMR24.
The detection and measurement of anti-HLA antibodies is accomplished using solid phase assays (Figure 2). Quantification is clinically relevant, as antibodies of greater intensity in vitro are potentially more cytotoxic in vivo. The presence of high-level anti-HLA antibodies (usually median fluorescent intensity [MFI] above 3,000–5,000) are considered potentially cytotoxic24.

Antibodies bind fluorescent-tagged antigens. A flow cytometer identifies anti-HLA antibodies and provides information on antibody strength and potential cytotoxicity.
However, not all high-intensity antibodies are detrimental to graft function and the fact that donor-specific antibodies have the ability to fix complement might be a superior way of signifying cytotoxicity25,26. The classical complement pathway is activated first by C1q binding to antibodies, as C1q is the first component of the pathway. After C1q activation, the complement cascade results in formation of the membrane attack complex and ultimately leads to cell lysis and death. In fact, antibodies with the ability to bind C1q are more likely to be cytotoxic25,27. The C1q assay is not currently available in all centers, so considering only antibodies that are strong binding by MFI after a 1:8 or 1:16 dilution may offer comparable information26.
Figure 3 illustrates one approach to the identification and quantification of antibodies against HLA and how management decisions are made in sensitized heart transplant candidates.
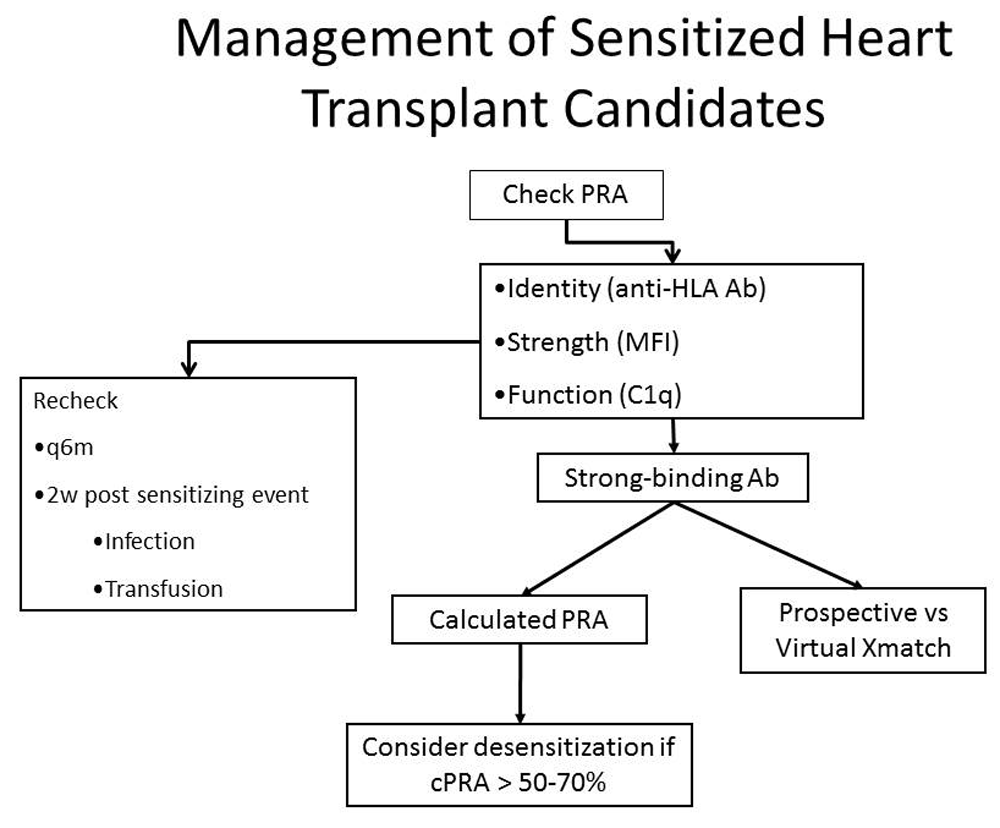
Sensitized patients are those with a positive panel reactive antibody (PRA) screen; we consider a PRA > 10% to be positive. The next step is to determine the identity and intensity of the anti-HLA antibodies. The results are used to determine the calculated PRA and the need for a virtual crossmatch. If the calculated PRA is above 50–70%, desensitization therapy may be used. This figure was reprinted with permission from 28.
Prior to transplantation, the detection of anti-HLA antibodies is important to avoid hyperacute rejection: one would avoid donors with HLA correlating with high-level anti-HLA antibodies in the prospective recipient. One way to prevent hyperacute rejection is a prospective crossmatch, in which the potential recipient’s serum and donor cells are mixed to evaluate for complement-dependent cytotoxicity. However, this restricts the donor pool based on the location of the candidate’s serum, and so decreasing the number of possible donors.
Thus, the virtual crossmatch has essentially replaced the prospective crossmatch. HLA correlating with the candidate’s high-level anti-HLA antibodies are recorded as “avoids” in the United Network of Organ Sharing (UNOS) database. In this manner, potential donors with such HLA are not considered. This method has proven feasible in HTx29.
The identity and the intensity score (given by Luminex single-antigen bead assay) of anti-HLA antibodies is also valuable information for deciding which potential heart transplant recipients require desensitization, as expressed as the calculated PRA (cPRA)24,30. The cPRA is the frequency of HLA defined as unacceptable in the donor population. For example, a HTx candidate with multiple anti-HLA antibodies might have a cPRA of 90%. This would mean that of all potential donors only 10% would be a match. cPRA highlights the fact that some high-level anti-HLA antibodies, the more common ones, will impact the ability to identify a suitable donor heart more than less common anti-HLA antibodies31. If the cPRA is above 50–70%, therapies to reduce antibody levels may be administered.
Management of the sensitized patient includes protocols that target antibodies by inactivation (intravenous immune globulin [IV Ig]32), removal (plasmapheresis), and reduced production (rituximab32) and bortezomib33). At our center, the desensitization process usually starts with a modified protocol built around one established for desensitization of kidney transplant recipients (Figure 4)32.
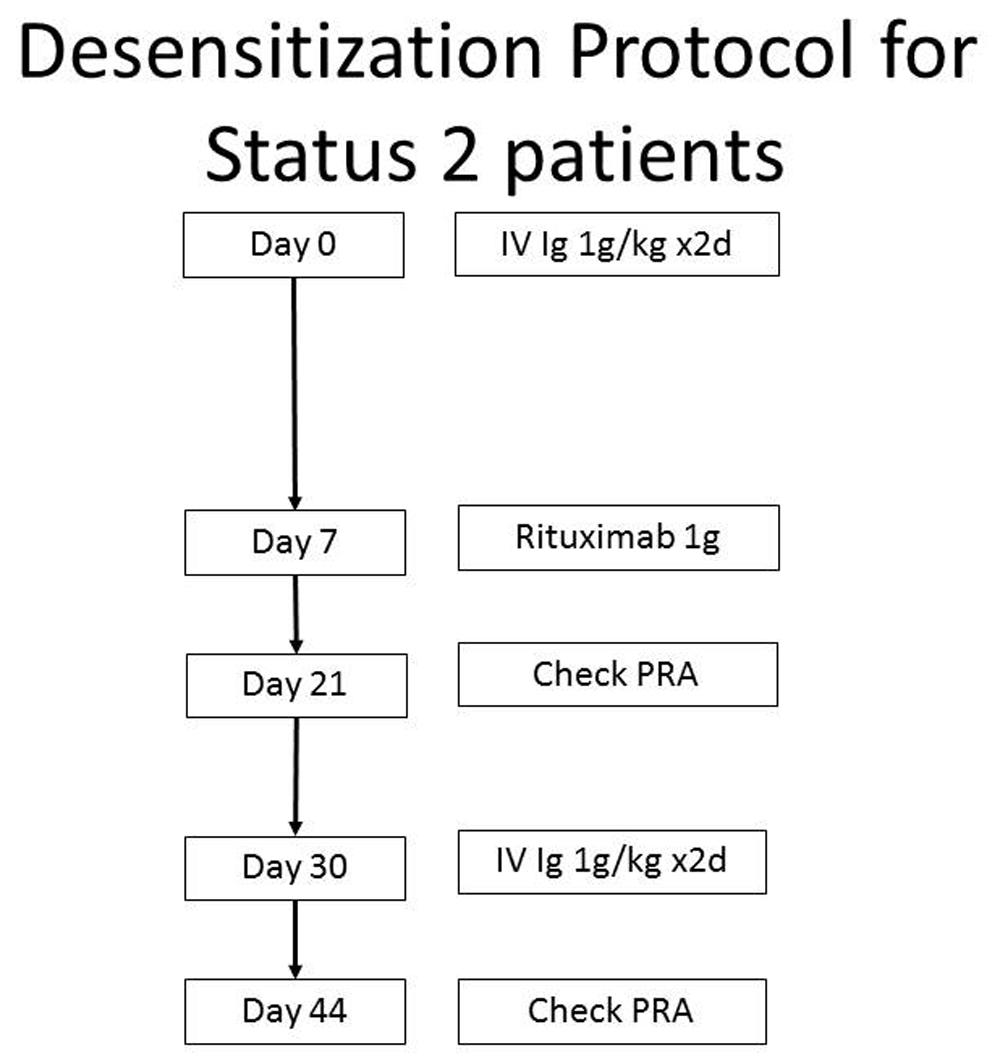
The treatment of circulating antibodies depends on the cPRA. Treatment is considered for those patients with cPRA >50–70%. This figure was modified from 32.
If the combination of intravenous immune globulin and rituximab is ineffective in decreasing the cPRA below 50%, or if swift desensitization is required (i.e. a patient is listed as status 1A), then one may use bortezomib, a proteasome inhibitor targeting plasma cells33. To increase effectiveness, bortezomib can be combined with plasmapheresis (Figure 5).
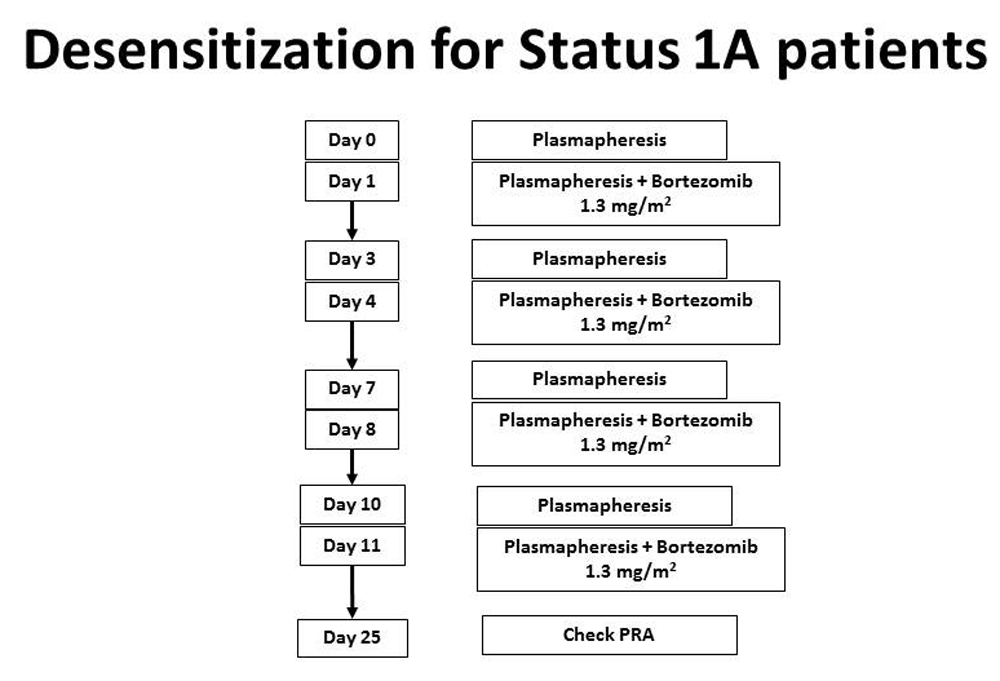
Bortezomib is used for Status 1A patients or those with antibodies that do not respond to IV Ig and rituximab. This regimen will lower antibodies more effectively. This figure was reprinted with permission from Kittleson MM, Kobashigawa JA. Management of the Highly Sensitized Patient Awaiting Heart Transplant. January 8, 2015. Available at: http://www.acc.org/latest-in-cardiology/articles/2014/12/22/17/07/management-of-the-highly-sensitized-patient-awaiting-heart-transplant-expert-analysis (accessed April 9, 2018).
Eculizumab. Pre-transplant interventions with IV Ig, rituximab, and bortezomib can reduce antibody levels so that it is possible to find an acceptable donor. However, for some patients such measures are not effective. Hyperacute rejection can still occur at the time of transplantation due to cytotoxic anti-HLA antibodies. This can happen if donor-specific antibodies were mistakenly classified as not cytotoxic by virtual crossmatch or if such antibodies were not present at the time banked blood was stored for a prospective crossmatch. Plasmapheresis may be used in the operating room at the time of transplantation in this setting. We have also found that eculizumab offers further insurance and protection against hyperacute rejection.
The monoclonal antibody eculizumab blocks the last component of the complement cascade. This cascade is triggered by antigen-antibody complexes and results in formation of the membrane attack complex and ultimately cell death. Eculizumab specifically binds to the terminal complement component 5 (C5), and thus eventually blocks the production of the terminal complement complex C5b-9. Eculizumab is FDA-approved for use in two complement-mediated conditions, paroxysmal nocturnal hemoglobunuria and atypical hemolytic-uremic syndrome. However, it also has benefit in sensitized kidney transplant recipients34.
The boundaries of HTx continue to expand to higher-risk candidates—older, on mechanical circulatory support, and with HLA antibody sensitization—and our goal remains to maintain favorable outcomes utilizing this scarce resource. The advances outlined here, from efforts to expand the donor pool, revision of the HTx allocation policy, newer ways to diagnose rejection, to developments in the detection and care of sensitized HTx candidates, will bring about improvements in survival and quality of life of end-stage patients who have HTx.
AMR, antibody-mediated rejection; cPRA, calculated panel reactive antibodies; ECMO, extracorporeal membrane oxygenation; HTx, heart transplantation; IV Ig, intravenous immune globulin; MFI, median fluorescent intensity; PRA, panel reactive antibodies.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 05 Jul 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)