Keywords
Bamboo leaves, Sodium hydroxide, Chemical treatment, FTIR, SEM
Bamboo leaves, Sodium hydroxide, Chemical treatment, FTIR, SEM
Bamboo is one of the most common plants available in Malaysia and is known for its multiple uses for construction, transportation and cultural purposes. About 70 species of bamboo (50 in Peninsular Malaysia, 30 in Sabah and 20 in Sarawak) can be found in Malaysia1. Being abundant throughout the whole country, bamboo can be treated as a source of income. Its leaf is claimed to have medicinal benefits among traditional practitioners2. Bamboo leaves, also known as Lophaterum, and bamboo shavings are commonly used as relievers for stomach aches, cooling effects and also help to counter the negative flow of qi3.
Asian populations include bamboo in their dishes, especially Chinese individuals. In China and Southeast Asia, bamboo had been used as source of food and medicine for a long time4. Bamboo grows one third faster than the fastest growing tree5 and some species can grow up to 60 meters high. Bamboo can grow up to 1 meter per day and therefore it is easily accessible in a minimal amount of time. The optimum age for bamboo to be harvested is at 3–5 years old. For thousands of years, bamboo had been part of human’s diet.
In addition, the Indian elephant and the Giant Panda of China also utilize bamboo leaves as their main food source, which become their exclusive meal. The skeletal system of the panda is incredibly strong, and flexible and it can be related to their diet of bamboo leaves, which contains a great amount of silica6. Usage of silica includes silica as dessicants, as a cement, and in the production of tires. Silica gel desiccants are used as pharmaceutical-desiccants/silica gels to promote drying by absorbing water vapor and gases, like oxygen and hydrogen in humidity. It is very important in pharmaceutical industries as humidity and moisture can ruin pharmaceutical products7. Silica has been utilized as a cement substitution, as silica exists as quartz in the silica sand. It has also been used as a fractional substitution of cement during solid development8. In tires, mixing silica with carbon improves the tire performance on the road9, as silica helps to provide flexibility through low-heat build-up.
Dracaena surculosa is a fast growing species of bamboo and is therefore preferable as an indoor plant. This species also prefers partial shade for growth. Exposure to bright lights may cause the plant to lose or shed its leaves. A full grown D. surculosa leaf could be as long as 7 – 12 cm long. The surface of the leaf is leathery and smooth with obvious patterns of yellow or gold spots (Figure 1).
Popular for its shoots, Gigantochloa albociliata is commonly planted and harvested as a food source and decoration. The shoots are edible and in Japan canned bamboo shoots are used. This bamboo species can easily grow in dry tropical mixed forest from low to medium elevations. The leaves are linear - lanceolate with the width of 15–20cm × 2–25cm (Figure 2). In the dry season, the leaves are shed10.
The abundance of bamboo in Malaysia may give a great advantage to the nation. However when harvested, not all parts of the bamboo are fully utilized, the leaves being especially discarded. This research aims to determine the organic compounds in bamboo leaves in two species, D. surculosa and G. albociliata after sodium hydroxide (NaOH) treatment. Along with sodium sulfide, NaOH is used to separate lignin from cellulose fibers in the craft process of making paper. Thus, this treatment is needed to separate lignin from fibers in order to identify the characteristics of leaves and was therefore used in the present study.
Sodium hydroxide (Quality Reagent Chemical [QREC], 1.0 M), nitric acid (QREC, 1.0 M) and toluene (QREC, 0.1 M) were utilized in this study. Bamboo leaves were collected with special permissions from Lundang Committee of Kg Lundang (Dracaena surculosa: 6°06'00.0"N 102°15'33.1"E) and Director of UMK Jeli Campus for UMK Agropark Kelantan (Gigantochloa albociliata: 5°44'46.0"N 101°51'58.3"E). All other chemicals and reagents used were of the highest commercially available purity.
The bamboo leaves from G. albociliata and D. surculosa (5 g sample) was stirred in 125mL of 1.0M NaOH for 8 hours using a magnetic stirrer. The solution was filtrated (filer paper grade 1, 11 µm) to separate the solute (bamboo leaves) and the aqueous solution (sodium silicate). The solution was then titrated with 1.0M nitric acid to obtain less than 3 pH in order to obtain the gel of suspended silica. Subsequently, this solution was centrifuged at 4000 rpm for 10 minutes. Then, the solution was left for 4 days to allow the silica gel to form. The silica gel were then filtered (filter grade 1, 11 µm) and extracted using 50mL of toluene. Finally, the solution obtained was dried over anhydrous sodium sulfate (QREC 1.0 M). This was then left overnight at room temperature. If any liquid remains, this should be collected and discarded. The final product will be analyzed using Fourier-transform infrared spectroscopy (FTIR). FTIR analysis is used to identify functional groups in a molecule by producing an infrared absorption spectrum. The spectrums were identified using Perkin Elmer Spectrum 100 FT-IR Spectrometer with range spectrum (wavelength): 4000–400/cm. Scan rate: 16, 32. Universal ATR sampling accessory (not using KBr) was employed to prepare the sample.
Each bamboo leaf sample was analysed using SEM to characterize the morphology of the specimen before and after treatment with NaOH. 30 g of each bamboo leaf was left untreated and ground to obtain a powder. In addition, 30g of bamboo leaves were treated with NaOH (30 mL) by stirring for 8 hours using a magnetic stirrer. The final solution was filtered using filter paper to obtain the solutes. The solutes were dried thoroughly before being ground into a powder to be analysed using SEM. The voltage used was between the ranges 1.5kV to 2.0kV. Secondary electron images were obtained.
During NaOH treatment, D. surculosa leaves exhibit a rapid change in colour and texture. The initial colour for this sample is light green and yellowish. After being stirred with NaOH, the colour started to change to dark brown, and after 8 hours of treatment, the colour turned to very dark brown. A reason for the rapid colour change is due to the thickness of the leaves, as any pigment inside the leaf would be quickly released as the leaves are thin. In addition, after 8 hours of treatment, the leaves almost completely dissolved into the solution of NaOH. This makes it hard for the leaves to be used in the next treatment of solvent extraction.
For G. albociliata leaves, there was a slower reaction during treatment of NaOH. After a few hours of treatment, the colour of the leaves started to change to dark green (original color light brownish green) and after 8 hours, the colour turns to almost black. In contrast to D. surculosa, the leaves did not dissolve into the solution.
FTIR spectra of changes in functional group of both untreated and treated (NaOH) bamboo leaves of G. albociliata is illustrated in Figure 3.
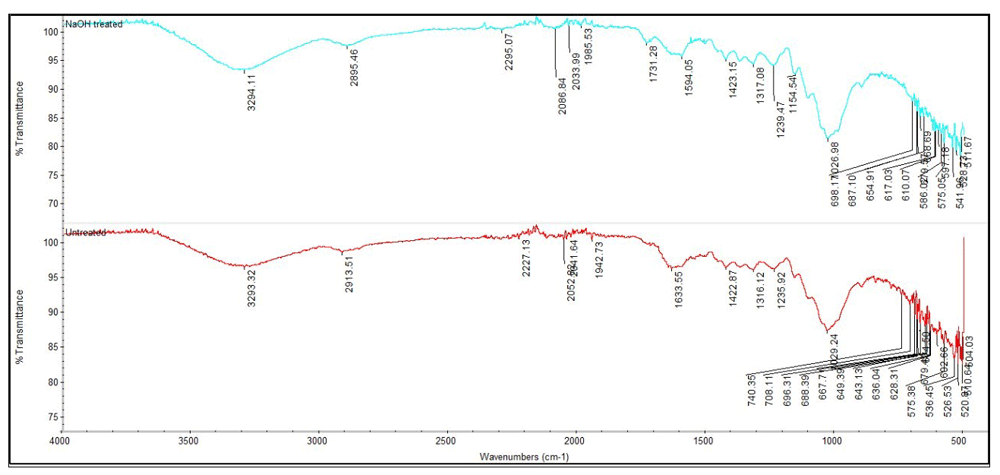
(Graphs are representative of 2 repeated experiments.)
The absorption band at 3293.32cm-1 indicates that there is an O-H stretching vibrations between cellulose and hemicellulose11. Meanwhile the 2913.51cm-1 for treated and untreated bamboo leaves demonstrate the presence of CH2 groups. The band also contains functional group of alkynes from the peak at 2913.51 cm-1. In accordance to the presence of this peak, it also indicates that before the treatment with NaOH, pectin and waxes are present in the bamboo leaves sample of G. albociliata12. Different results are achieved with bamboo leaves treated with NaOH where the peaks have almost disappeared. This shows that hemicellulose is present and is gradually removed from the sample during treatment. The lists of functional groups are shown in Table 1.
| Before treatment with NaOH | After treatment with NaOH | ||
|---|---|---|---|
| Peak | Functional group | Peak | Functional groups |
| 3293.32 | OH | 3294.11 | OH |
| 2913.51 | CH | 2913.51 | CH |
| 2227.13 | Alkenes ( C double bond) | 2295.07 | Cannot detect |
FTIR spectra of changes in functional group of untreated and treated D. surculosa leaf samples using 1.0M NaOH is illustrated in Figure 4.
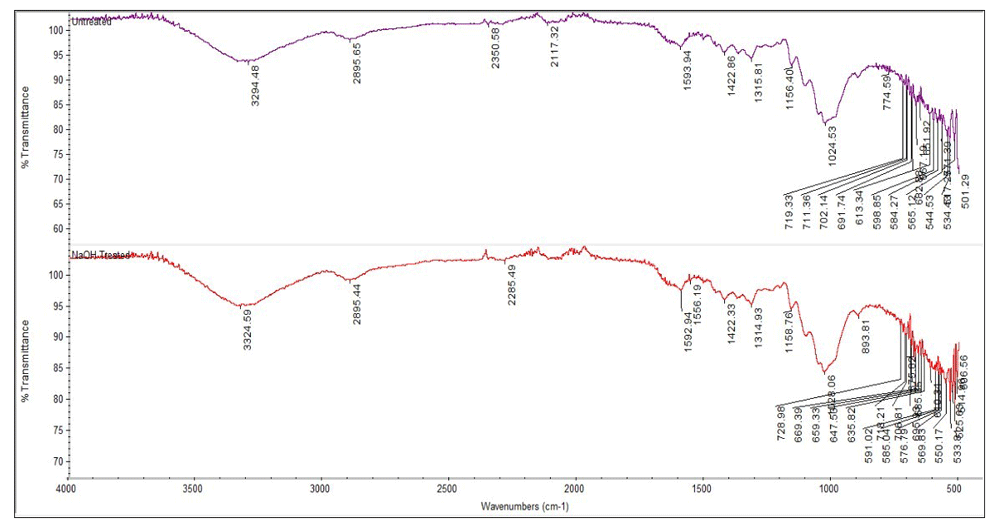
(Graphs are representative of 2 repeated experiments.)
The absorption band at 3294.48 cm-1 in D. surculosa leaves indicates that there is a O-H stretching vibrations between cellulose and hemicellulose13. Whereas the 2885.64 – 2895.44 cm-1 for treated and untreated bamboo leaves sample show the presence of CH2 groups. The band contains functional group of alkynes with triple bonds from the peak at 2117.32.51 cm-1. Different results are achieved with bamboo leaves treated with NaOH where the peaks had disappeared. The list of functional groups are shown in Table 2.
The study of morphology requires SEM analysis before and after treatment is done, as the treatment may affect the morphology of the species14. Figure 5 shows a 400x magnification of G. albociliata leaf surface before treatment with NaOH.
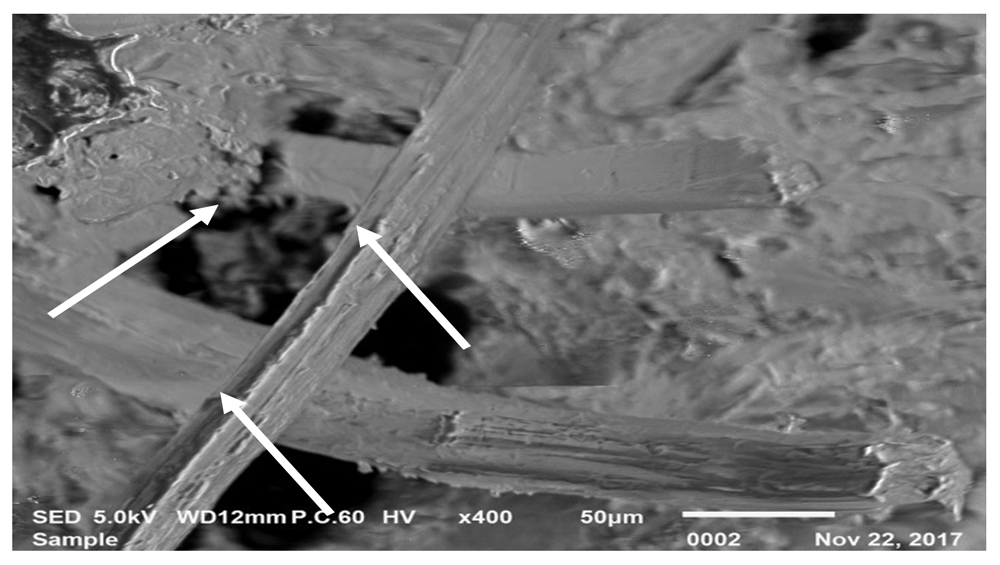
White arrows shows the fiber bonding in the leaf.
From this figure, it can be observed that the bonding between fibers in the leaf are still intact as shown by the arrows in Figure 5. Fibers play an important role in improving strength, providing higher initial modulus and reducing extensibility by improving the interfacial adhesion between them15.
From Figure 6, it can be observed that the leaves had no particular shape and the morphology changes completely after being treated with NaOH. The degradation of hemicellulose may occur during treatment, which causes the leaf to be dissolved in the solvent itself. From this observation, it can be concluded that NaOH is one agent that can degrade the molecular structure of bamboo leaves. Furthermore, high concentration of NaOH used in this test (1.0M) may contribute to the rapid degradation of the hemicellulose. Another observation using SEM analysis shows the structure of the fibres are in non-uniform shape.
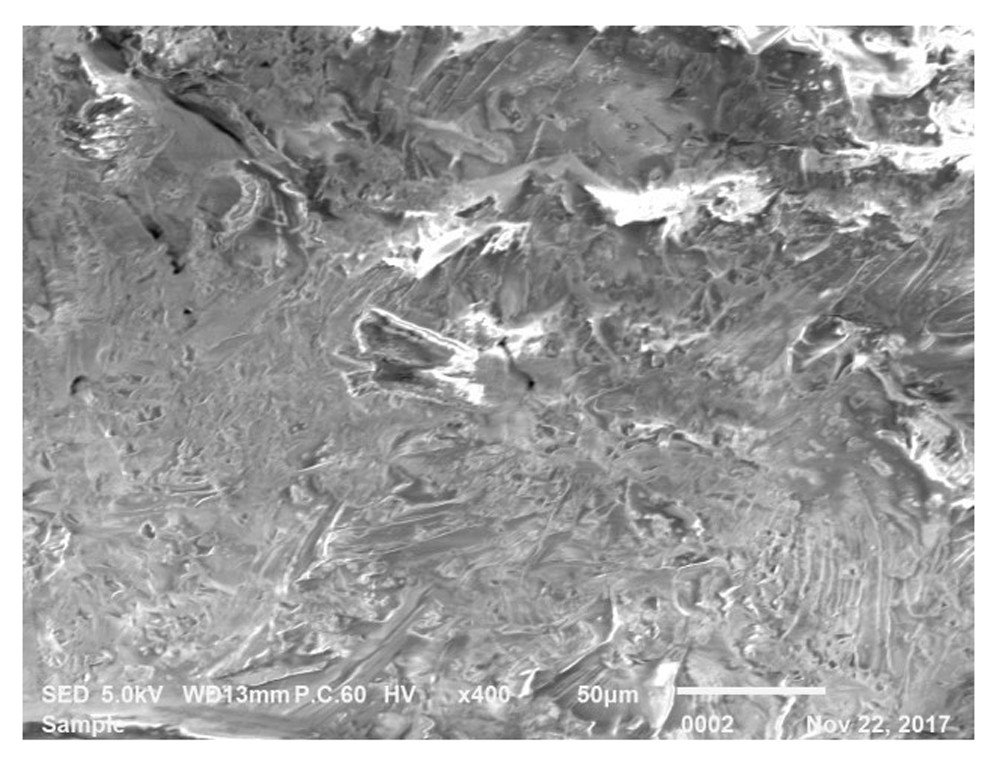
The leaves of untreated D. surculosa still have bonds between the hemicelluloses. After the grinding process, the leaves are crushed but remains in solid form, as seen in Figure 7, and are not powdery like G. albociliata.

The white ring shows that the fiber bond which is intact but not clearly shown.
After treatment with 1.0M NaOH, the bamboo leaves become partially dissolved into the solvent, as can be seen in Figure 8. The final colour of the solution also changes to dark brown. This change of colour is caused by the removal of hemicellulose in the leaf itself16.
From the FTIR analysis, the functional group found in the specimens can be identified based on the presence of the bands in the graphs. It can be concluded that the process of treatment and extraction had contributed to the organic compounds present in the samples of different species6. Among the two samples, G. albociliata and D.surculosa, the initial morphology is different in two factors, colour and appearance. The initial colour of G. albociliata and D.surculosa is yellowish green and dark green, respectively. However after the treatment, the colour becomes almost the same. As for the SEM analysis, the treatment shows the morphology of the bamboo leaves completely changes due to the treatment with NaOH. Due to change in colour of NaOH, it can be concluded that the process of hemicellulose removal had occurred during the treatment. Bamboo is truly interesting to study as it has a unique anatomy and a superproductive behavior. Rhizomes are an essential part of bamboo anatomy. This study will be able to provide new data regarding the characteristics of G. albociliata and D. surculosa bamboo leaves after NaOH treatment
Dataset 1: Replicates of FTIR analysis; bamboo leaves after NaOH treatment. DOI, http://dx.doi.org/10.5256/f1000research.15036.d20885917
We thank UMK research short term grant SGJP for funding (number R/SGJP/A13.00/00692A/001/2018/000498).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical Chemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Jul 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)