Keywords
Core-Binding Factor Subunit-α, Gingival Stromal Progenitor Cells, Osteogenic Differentiation, Platelet Rich Fibrin, Sox9.
Core-Binding Factor Subunit-α, Gingival Stromal Progenitor Cells, Osteogenic Differentiation, Platelet Rich Fibrin, Sox9.
Dental caries represents a major global dental public health problem because of their high prevalence. The World Health Organization reported that almost 90% of people worldwide suffered from caries1. Basic Health Research of National Health (RISKESDAS) in 2013 reported that 93,998,727 Indonesians, 53.2% of the population, suffered from active caries2. Dental caries must be treated appropriately because, if neglected, they become so severe that the affected teeth must be extracted. Indeed, the most common cause of tooth loss is dental caries3. Populations experiencing low socioeconomic conditions demonstrate higher prevalence and extent of tooth loss because of extremely limited access to dental treatment4.
Tooth extraction has been the most common form of dental treatment performed in Indonesia that can lead to bone defects. RISKESDAS statistics dating from 2014 indicated that treatment involving tooth extraction reached as high as 79.6% of cases5. A previous study of tooth extraction-related complications revealed the prevalence of fractures (31.82%), bleeding (4.54%) and swelling (2.27%)6. Tooth extraction can lead to alveolar bone resorption and the destruction of alveolar bone components. Moreover, it may lead to resorption of the jawbone7. Tooth extraction followed by buccolingual and apicocoronal dimension reduction of the alveolar ridge at the edentulous site might be performed due to bone defects8.
Alveolar bone defect regeneration has long represented a challenge in the field of dentistry. Various efforts have been made to accelerate bone regeneration, such as using bone grafts. The most current treatment performed in relation to the alveolar bone involves the use of platelet rich fibrin (PRF). PRF materials encouraging bone regeneration therapy have significantly improved the clinical outcomes stemming from the treatment of infrabony defects. PRF has achieved this through the maintaining of space for tissue regeneration by inducing an osteoinductive and osteoconductive effect in the alveolar bone defect area9.
Nowadays, alveolar bone defect treatment involving PRF using stromal progenitor cells (SPCs) is becoming increasingly widespread. SPCs have the advantage of being able to repair and regenerate various organs and tissue, and have been considerably used in bone tissue engineering, which offers encouraging solutions for bone regeneration10,11. SPCs are non-hematopoietic stromal cells. They have multipotent capabilities, including immunomodulators and immunoregulators, paracrine, autocrine action, and migrate directly to the tissue initiating healing and regeneration making SPCs particularly suitable for regenerative medicine development12–14.
The orofacial region is a unique and rich source of SPCs. Those contained in the oral cavity and tooth tissue represent an emerging interesting and topical object for investigation because isolating progenitor cells from the oral tissues can be achieved with minimal invasive procedures compared to bone marrow mesenchymal stem cell (BMSC) obtainment. The utilization of progenitor cells from the oral cavity is still rarely studied and applied. However, it is potentially advantageous for tissue regeneration and, therefore, merits further investigation15.
The SPCs that are potentially useful as part of regenerative alveolar bone therapy are gingival stromal progenitor cells (GSPCs) derived from hyperplastic gingival tissue (gum overgrowth) by means of a gingivectomy. GSPCs have phenotypic characteristics and abilities similar to those of BMSCs. GSPCs possess self-renewal capabilities and also demonstrate the specific ability to regenerate into alveolar bone when transplanted into immunocompromised mice. GSPCs also specifically induce bone matrix formation in lamellar structures by recruiting host cells11,15–17. The osteogenic ability of GSPCs needs to be explored for further application and therapy.
During skeletal formation, master transcription genes such core-binding factor subunit-α (CBF-α1) and Osterix have been identified18. However, their specific and distinct roles in various tissue types are still unclear. Sox9 is well known as a master gene regulator during chondrogenic differentiation, while CBF-α1 plays an important role during osteogenic differentiation. GSPCs, as osteoprogenitors and chondroprogenitors, express Sox9 and Runx2 during skeletal formation condensation. There is also a direct interaction between Sox9 and CBF-α1, which inhibits Sox9 activity18. Sox9 inhibitory effect on osteoblast maturation through CBF-α1 is an essential mechanism for osteo-chondroprogenitor fate determination19.
In order for GSPCs to differentiate and proliferate optimally they require growth factors (GFs), various varieties of which are shown to promote osteogenic differentiation of SPCs in vitro. PRF is predicted to be combined to promote SPCs osteogenic differentiation and ensure mineralization in vitro19. PRF can be easily produced by centrifuging without anticoagulants. PRF is rich in GFs consisting of platelet derived growth factor-β (PDGF-β), transforming growth factor-β1 (TGFβ-1), vascular endothelial growth factor (VEGF) and insulin growth factor (IGF-I). PRF provides an effective scaffold to facilitate osteogenic differentiation of GSPCs20–23.
The osteogenic differentiation of GSPCs can be detected by various osteogenic marker expressions, such as CBF-α1 expression. The observed osteogenic markers of GSPCs are CBF subunit-α1 (CBF-α1) and Sox924,25. Nonetheless, there is insufficient information regarding Sox9’s role in osteogenesis of GPSCs in vitro. A study conducted by Stockl et al. mentioned that Sox9 plays a positive proliferative role in inhibiting and delaying osteogenic differentiation in rat SPCs26.
The hypothesis of the current study is that GSPCs cultured in PRF can increase the CBF-α1/Sox9 expression ratio during osteogenic differentiation. Furthermore, a second objective was to analyze GSPCs cultured in PRF osteogenic differentiation predicted by CBF-α1/Sox9 expression ratio.
This study received ethical clearance relating to animal subjects from the Ethics Research Committee, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia (number 289/HRECC.FODM/XII/2017). The research was conducted at an experimental laboratory within the Stem Cell and Tissue Engineering Development Centre, Universitas Airlangga.
The research was fully experimental with a post-test only control group design. Sample groups were selected by means of simple random number sampling. Each animal was assigned a unique number, which were picked out of a hat by a blindfolded researcher.
The subjects consisted of male Wistar rats (Rattus norvegicus; n=4), who were adapted to the environment for 7 days. Wistar rats were obtained and cared for at the Stem Cell Animal Laboratory, Universitas Airlangga. All animals were housed in polycarbonate cages, subjected to a 12-hour light-dark cycle at the constant temperature of 23°C, and fed a standard pellet diet (expanded pellets; Stepfield, UK) with tap water ad libitum at a temperature of 22°C±2°C.
GPSCs were isolated from the lower gingival tissue of four 1-month old, healthy, mean weight = 250g, male rats through a gingivectomy, before the rats were euthanized with doses 60mg/body weight of ketamine and xylazine. Animal suffering was reduced when removing the GPSCs using rodent’s anesthesia (intramuscular injection at 0.05–0.1ml/10g body weight rodent anesthesia: ketamine, xylazine, acepromazine, and sterile isotonic saline; Sigma Aldrich, USA) following Duan et al’s method23.
GPSCs was passaged every 4–5 days following Rantam et al’s SPCs culture method27. GSPCs in passage 3–5 were cultured in five M24 plates (Sigma-Aldrich) (N=108; n=6/group) until Day 7, Day 14 and Day 21 in three different culture mediums (control negative group, control positive group and treatment group; see below for details).
Sample size (n=4 for GPSCs isolation; n=36 for PRF isolation) was based on Lemeshow's formula to determine minimum sample size
A different population of rats were used for PRF isolation (n=36; 36 month old; mean weight = 250g). These male Wistar rats were maintained as above. Blood was aspirated through the left ventricle of each animals’ heart, after anesthesia had been administered by injection using a 60mg/body weight dose of ketamine and a 3mg/body weight dose of xylazine (Sigma Aldrich). 1.5ml of blood was aspirated using a 3ml disposable syringe and then inserted in a vacutainer tube without an anticoagulant before being centrifuged at 3000 rpm/min for 10min (Kubota, Tokyo, Japan). The centrifuging was performed by inserting two balance tubes containing water with the same weight as the tube of blood. When the tube is removed from the centrifuge, three layers will appear that are divided into three sections; the lower section consists of red blood cells, the middle section contains PRF and the upper section is formed of acellular plasma. The PRF was then isolated after which the PRF was cut into small pieces using sterile scissors and inserted into each culture plate of the treatment group22,28,29.
The analysis was conducted on three groups, consisting of two control groups and one experimental group.
GSPC treatment group: GSPCs were cultured with PRF and containing ITS plus, 2mM L-glutamine, 100μg/ml sodium pyruvate, 0.2mM ascorbic acid-2 phosphate, dexamethasone 10-7 M (GeneTex, Taiwan), 10ng/ml TGF-β3 and high-dose glucose-Dulbecco's Modified Eagle Medium (DMEM-HG) (Sigma Aldrich).
Positive control group: GSPCs were placed on an osteogenic medium culture plate of ITS plus, 2 mM L-glutamine, 100μg/ml sodium pyruvate 0.2mM ascorbic acid-2 phosphate, dexamethasone 10-7 M (GeneTex).
Negative control group: GSPCs were cultured with αModified Eagle Medium (αMEM) (Sigma Aldrich).
Every three days, every group cell medium was replaced. Osteogenic differentiation was evaluated on Day 7, 14, 21 culture cells groups16.
GSPCs cultured cells were coated with coverslips and, after incubation at 37°C for 1 - 2 hours, were fixed using 10% formaldehyde for 15 min. The coverslips were then rinsed four times with PBS and dried for several minutes. The cells were blocked with PBS and FBS 1% for 15–30 minutes and washed with PBS four times. The samples were then examined following immunocytochemical staining by indirect technique using a 3.3'-diaminobenzidine stain kit (Pierce DAB Substrate Paint Kit 34002, ThermofisherTM, Waltham, MA, USA) and monoclonal antibodies (Santa Cruz Biotechnology, Dallas, TX, USA): anti-CBF-α1 (mouse monoclonal; sc-101145) and anti-Sox9 (mouse monoclonal; sc-166505). CBF-α1 and Sox9 expression was read using a light microscope (CX22 Binocular, Olympus) at 200x magnification. Every cell expressing CBF1-α or Sox9 in one field was examined three times by three experts (WR, EH and FAR) and the mean was then calculated27,30,31.
The data obtained was analyzed using ANOVA continued with Tukey HSD test (p<0.05) based on a Saphiro-Wilk normality test and a Levene's variance of homogeneity test (p>0.05). Data were analyzed using SPSS version 20.0 (IBM SPSS, Chicago, USA).
The experiments were replicated 3 times (n=54). The data was then duplicated (n=108) using an estimation formula and SPSS (see Supplementary File 1 and Supplementary File 2)32.
The highest average CBF-α1 expression was in the treatment group on Day 7, whereas the lowest was in the control (-) group on Day 21 (Figure 1 and Figure 2). Sox9 expression had the highest mean value in the treatment group on Day 7, while its lowest value was in the negative control group on Day 21 (Figure 3 and Figure 4).
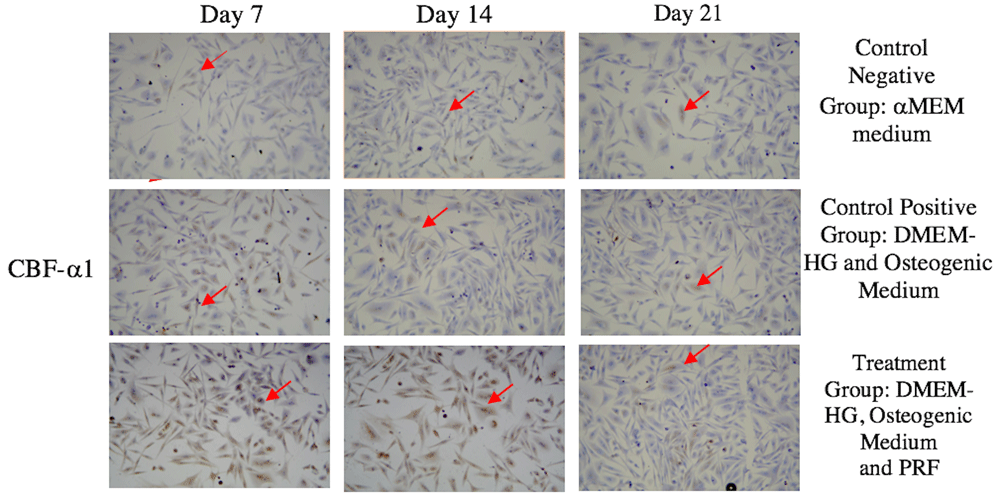
(A-C) CBF-α1 expression in the negative control group; (D-F) CBF-α1 expression in the positive control group; (G-I) CBF-α1 expression in the treatment group. CBF-α1 expression in GSPCs was observed on Days 7, 14 and 21. Positive CBF-α1 expression is highlighted in brown (red arrow) following an examination at 200x magnification (n=1).

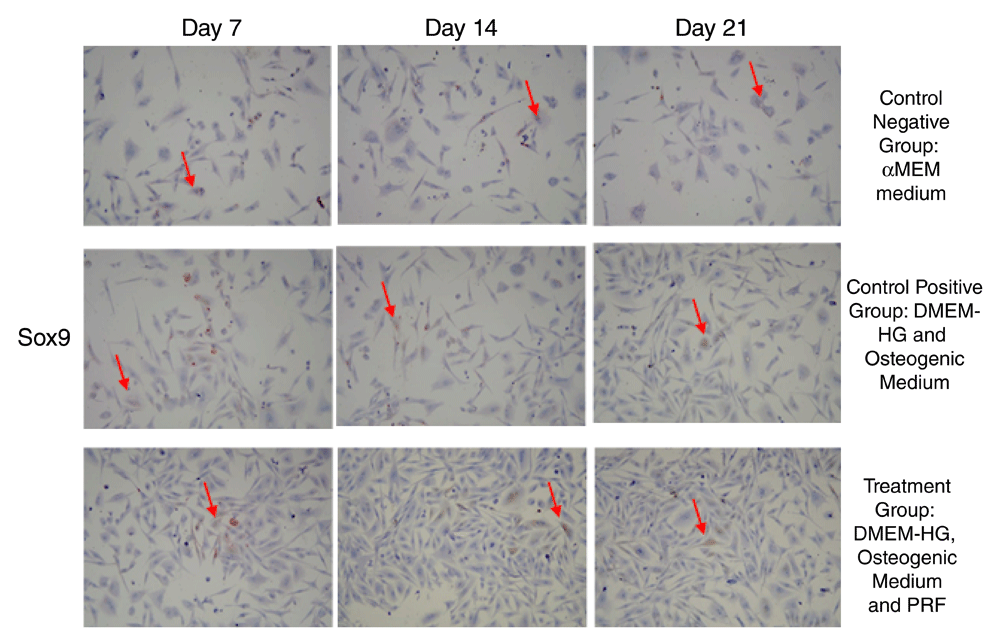
(A-C) Sox9 expression in the negative control group; (D-F) Sox9 expression in the positive control group; (G-I) Sox9 expression in the treatment group. Sox9 expression in GSPCs was observed on Days 7, 14 and 21. Positive Sox9 expression is highlighted in brown (red arrow) following examination at 200x magnification (n=1).
The treatment group recorded the highest CBF-α1/Sox9 ratio (16.00±3.000/14.33±2.517/) on Day 7 while the lowest CBF-α1/Sox9 ratio (3.33±1.528/3.67±1.155) was registered by the control negative group on Day 21 (Table 1). The data obtained was normal with homogeneous distribution (p>0.05). There was significant difference between CBF-α1 and Sox9 expression in each group (p<0.05) (Supplementary Table 1 and Supplementary Table 2).
| Day | CBF-α1 expression | Sox9 expression | P-value* | ||||
|---|---|---|---|---|---|---|---|
| Negative control group | Positive control group | Treatment group | Negative control group | Positive control group | Treatment group | ||
| 7 | 9.00±2.000 | 11.33±1.528 | 16.00±3.000 | 5.67±1.155 | 11.00±1.00 | 14.33±2.517 | 0.00 |
| 14 | 7.33±1.528 | 9.33±0.577 | 11.67±2.082 | 2.67±0.577 | 7.33±1.528 | 11.33±1.528 | 0.00 |
| 21 | 3.33±1.528 | 8.33±1.155 | 10.67±1.528 | 2.33±1.155 | 6.00±1.000 | 9.00±2.000 | 0.00 |
GSPCs cultured in PRF expressed CBF-α1 strongly. In this study, the highest CBF-α1 expression was recorded by the treatment group on Day 7, with significant difference between groups. The CBF-α1 expression declined between Day 14 and Day 21. The results of this study were in line with the research by Zou et al., which suggested that CBF-α1 expression is used to detect the osteogenic ability of SPCs using yellow fluorescent protein33.
CBF-α1 is a master key gene transcription factor associated with osteoblast differentiation, which initiates temporally and spatially controlled osteogenesis. Disturbances to CBF-α1 result in obstacles to bone formation because osteoblast differentiation cannot occur. Loss of CBF-α1 expression gene function in the early stages will interfere with osteogenic differentiation and homeostasis in bone development. CBF-α1 is often expressed strongly between Day 7 and Day 1423,25. Osterix and CBF-α1 periodically regulate osteoblast differentiation processes34,35. A study conducted by Loebel et al. showed that CBF-α1 expression increased on Day 7, while Duan et al.’s study demonstrated that CBF-α1 expression increased on Day 12 as detected by RT-PCR19,23. Such findings differed from the results of this study due to the contrasting methods and samples employed, but there were similarities in that CBF-α1 was an early marker of osteogenic differentiation.
CBF-α1 plays an important role in the early stages of BMSCs differentiation into preosteoblasts. CBF-α1 is generally a preliminary regulator and Osterix is a regulator activator during osteoblast differentiation. Both of these osteoblastogenic coding genes are stimulated and regulated by various signaling pathways, such as the canonical Wnt signaling pathway and bone morphogenetic protein (BMP). Wnt/Cytosolic β-catenin stimulates osteoblastogenesis through the activation of osteogenic transcription factors CBF-α1 and Osterix36. CBF-α1 is known as an important regulatory gene during osteogenic development by enhancing specific osteoblastic differentiation by inducing osteogenic extracellular matrix gene expression during osteoblast maturation, such as collagen-Iα, alkaline phosphatase, and osteocalcin33.
In the present study, the GSPCs cultured in PRF stimulates CBF-α1 expression because PRF is rich in various GFs, such as TGFβ-1, PDGF, IGF, VEGF, FGF, EGF, and HGF. PRF promotes migration, proliferation and differentiation of mesenchmymal stem cells as well as neovascularization and collagen synthesis. PRF also promotes, accelerates and improves the quality of soft and bone tissue regeneration23. According to Li et al., PRF significantly promotes the induction of mineralization of progenitor cells in alveolar bone, and endogenous stem cells present in the dental tissue that increases exclusively in CBF-α1 expression37,38.
Interestingly, GSPCs cultured PRF in this study increased Sox9 even in an osteogenic culture medium with significant difference with the control groups. In this study, the highest Sox9 expression occurred in the treatment group on Day 7. The results of this study were supported by those of a study by Sumarta et al., which stated that SPCs cultured in PRF stimulate Sox9 expression22. Sox9 expression showed a positive expression, thereby establishing the role of Sox9 during bone formation. In a knockout Sox9 animal model, osteogenic differentiation was also delayed39. Significantly, recent genetics studies stated that Sox9 in SPCs could eventually differentiate into osteoblasts40. Therefore, the inhibitory effect of Sox9 on osteoblastic and chondrocyte maturation via repression of CBF-alpha1 function is an essential mechanism for osteo-chondroprogenitor cell fate determination41.
In this study, GSPCs-cultured PRF regulated and stimulated both CBF-α1/Sox9 expression ratio on Day 7 with significant difference between groups. The interaction and cooperation between CBF-α1/Sox9 is a mandatory master transcription gene for cartilage and bone development18. As Sox9 inhibited and downregulated CBF-α1 on Day 7, it may be even more sensitive to predict osteogenic differentiation ability of SPCs. Furthermore, while Sox9 expression was downregulated, osteogenic differentiation ability was stimulated during early osteogenic differentiation in vitro. Nevertheless, CBF-α1/Sox9 expression ratio on Day 7 could be used to predict the osteogenic differentiation ability of GMSCs, suggesting a balance between CBF-α1/Sox9 in the earlier regulatory bone formation and regeneration19,41.
GSPCs cultured in PRF increased CBF-α1/Sox9 expression on Day 7. GSPCs cultured in PRF possessed potential osteogenic differentiation ability as predicted by the CBF-α1/sox9 expression ratio. CBF-α1/Sox9 expression constitutes a promising future in vitro screening method employed to detect the earliest osteogenic differentiation of SPCs. Further study is required to analyze any association with CBF-α1/Sox9 expression ratio in vivo.
Dataset 1: Raw results for CBF-α1 and Sox9 expression for all time points for all treatment groups (N=108; n=6/group). DOI, 10.5256/f1000research.15423.d21063842
Dataset 2: Raw image data. DOI, 10.5256/f1000research.15423.d21063943
The research was funded by the Progam Menuju Doktor Sarjana Unggul (PMDSU) Batch III of the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (Kemenristekdikti RI) (letter of appointment agreement number, 1035/D3/PG/2017; grant number 2146/D3/PG/2017).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank the Postgraduate School, Department of Dental Medicine, Faculty of Medicine, Stem Cell Research and Development Centre, Universitas Airlangga for its support of the research reported here.
Supplementary Table 1. Tukey HSD multiple comparison between groups of CBF-α1.
Click here to access the data.
Supplementary Table 2. Tukey HSD multiple comparison between groups of Sox9 expression.
Click here to access the data.
Supplementary File 1: Estimation resume of CBF-α1 expression.
Click here to access the data.
Supplementary File 2: Estimation resume of Sox9 expression.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biological tooth movement
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 25 Jul 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)