Keywords
Burn infection, Pathogenic bacteria, Antimicrobials susceptibility patterns, ESBL.
Burn infection, Pathogenic bacteria, Antimicrobials susceptibility patterns, ESBL.
Burn infection caused by pathogenic bacteria is one of the most common hospital problems worldwide, particularly in developing countries1. Fire leads to skin destruction and simultaneous suppression of both humoral and cellular immune system subsequently resulting burn infection2. Complications of burn infection are responsible for more than 70% of death cases among inpatients with burns3. These infections mainly caused by multi-drug resistant gram-negative and gram-positive bacteria such as Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K.pneumoniae) and Staphylococcus aureus (S.aureus)4,5. Non-sterile burns halls and duration of patients stay in hospital in addition to the surface area of burned skin, are the most important factors related to the increase of persistent and multiplication of pathogenic bacteria in the burned areas6,7. Multi-drug resistant (MDR) bacteria is one of the most common pathogens causing burn infection in hospitalized patients worldwide8,9. These pathogens are resisting to at least three different classes of antimicrobials such as, penicillin’s, beta-lactams, cephems, 3rd and 4th generation cephalosporins, aminoglycosides, tetracyclines and quinolones, and is becoming one of the most dangerous health issues in hospitals10. In addition, extended-spectrum β-lactamase (ESBL)-producing bacteria are considered as a potent pathogens due to it their resistance to a wide range of antimicrobials like, cefotaxime, ceftriaxone and ceftazidime, that lead to difficulty in the treatment of most infections such as burn infection and urinary tract infection11,12. Burn infection is characterized by difficult healing due to administration of unsuitable treatment, long stays in hospital and the contaminates of hospital environments lead to the emergence of new multi-drug resistant bacterial isolates causing dangerous complications such as, bacteremia, septicemia and death13,14. Therefore, we must pay attention to all safety standards in hospitals, especially in burns wards through sterilization, performing antimicrobial susceptibility test on all pathogenic bacteria isolated from burn infections, and keeping the burned skin in sterile conditions to prevent the emergence of these pathogens. According to the above, the aim of this work was to investigate of the prevalence of multi-drug resistant bacteria and extended-spectrum β-lactamase-producing bacteria isolated from inpatients with burn infection in Al-Najaf central hospital in Al-Najaf City, Iraq over three years, from January 2015 to December 2017 to increase our understanding of the most prominent bacteria and their resistance to different antimicrobials to prevent the emergence of these isolates in the future.
We confirm that we received approval for this study including: patient’s swabs and consent from the participants. All swabs were taken by physician and consent by the hospital treatment and care team responsible and then handed the samples over to us. All swabs were provided from the participants physician in Al-Najaf central hospital in Al-Najaf City, (Burns Department). All swabs were immediately transported to the Laboratory of Microbiology in Faculty of Science, University of Kufa to process. Note: Each swab was labeled with the following items: age, sex, duration of stay in the hospital after burning. Al Najaf Central Hospital is part of the University of Kufa and therefore written approval was not sought as there is a pre-existing agreement between the university and hospital regarding clinical sample collection sample collection. Oral consent to take swabs was taken from each patient.
Patients will be considered eligible for registration into this study if they fulfill all the inclusion criteria and none of the exclusion criteria as defined below.
1- Patients (Male or female) at least more than 18 years old.
2- Patients should have sufficient capacity for informed consent.
3- Patients should don’t have any other infections.
This is a cross-sectional descriptive study performed in Al-Najaf central hospital in Al-Najaf City, Iraq, from January 2015 to December 2017. A total of 295 swabs (emulsion with normal saline) were collected from the burned area of hospitalized patient with burn infections (2nd degree, shown the signs of infection during the change of dressings), ages ranges 18-45 years old (males and females), 3 swabs were taken from each patient at 5, 10 and 15 days of stay. Immediately, all collected swabs were incubated with brain heart infusion broth (Oxoid™, USA, CM1135R) for 24h at 37°C to encourage bacterial growth and then streaked onto blood agar (Oxoid™, USA, CM0055B) using a swab (Himedia, India, PW1210G) and chocolate agar (Oxoid™, USA, R01293) surface and incubated aerobically at 37°C for 24-48 h. All emerged bacterial isolates were identified according to colony morphology and standard microbiological tests such as; colony morphology, blood hemolysis onto blood agar surface (Oxoid™, USA, CM0055B), gram stain, oxidase test, catalase test, imvic test, motility test, coagulase test, growth on MacConkey agar (Oxoid™, USA, R061322) and Mannitol salt agar (Oxoid™, USA, CM0085B)15.
Antimicrobials susceptibility testing was performed by disc diffusion method according to Kirby-Bauer method onto Mueller Hinton agar (Oxoid™, USA, PO5007A) surface16. Fourteen different antimicrobial discs were used in this study provide from OxoidTM, USA as follow: penicillin 10IU (P) (CT0043B), amoxicillin 25µg (AX) (CT0161B), Amoxiclav 30µg (AMC) (CT0538B), ceftriaxone 30µg (CRO) (CT0417B), cefotaxime 30µg (CTX) (CT0166B), ceftazidime 30µg (CAZ) (CT0412B), gentamicin 10µg (GM) (CT0024B), tobramycin 10µg (TM) (CT0056B), amikacin 30µg (NA) (CT0107B), ciprofloxacin 5µg (CIP) (CT0425B), imipenem 10µg (IMP) (CT0455B), Streptomycin 10µg (S) (CT0047B), erythromycin 30µg (E) (CT0021B), tetracycline 30 µg (TE) (CT0054B). The diameters of inhibition zones (mm) were measured using a caliper measure each zone with the unaided eye, and compared with clinical and laboratory standards institute (CLSI) guideline 201717. Any bacterial isolate was resistance to at least three different antimicrobials classes considered as MDR, if any bacterial isolate was resistance to all antimicrobial classes except two or three antimicrobial classes considered as extensive-drug resistant (XDR) and when any bacterial isolate was resistance to all antimicrobials class considered as pan-drug resistant (PDR)17.
All S.aureus isolates growth was adjusted according to turbidity of standard McFarland tube 0.5 (measured by Vis-Nir spectrophotometer, Biobase, UK, bk-S410). All isolates were streaked onto Mueller Hinton agar (Oxoid™, USA, PO5007A) surface supplemented with 4% NaCl. Five µg of methicillin disc (Oxoid™, USA, CT0159B) was placed at the surface of Mueller Hinton agar and incubated aerobically at 37°C for 24h. All S. aureus isolates that were resistant to methicillin with diameter of inhibition zone < 17 mm were considered as methicillin resistant S.aureus (MRSA), while those isolates with diameters of inhibition zone ≥ 17 mm considered methicillin sensitive S.aureus (MSSA)18.
This test was performed according to modified double disc synergy test (MDDST)19 as follows: all bacterial isolates (turbidity was adjusted according to McFarland tube 0.5) were streaked by sterile swab (Himedia, India) onto Mueller Hinton agar (OxoidTM, USA) surface, AMC disc 30µg was placed in the center of agar plate, CRO 30µg, CTX 30µg and CAZ 30µg were placed around AMC disc 30µg (15 mm from center to center). All plates were incubated aerobically at 37 °C for 24h. Any increase in the inhibition zone towards AMC disc 30µg was considered as positive for the extended spectrum beta-lactamase.
Percentages were used in this study to compare between the prevalence of pathogenic bacteria and their resistant to antimicrobials using Graphpad-prism V.10 computer software.
Of the 295 burn swabs, 513 different bacterial strains were isolated, 335 isolates (65.3%) were gram negative bacteria and 178 isolates (34.7%) were gram positive bacteria (Figure 1). Pseudomonas aeruginosa was one of the most common bacteria causing burn infection, 142 isolates (27.6%), followed by methicillin resistant S.aureus 106 isolates (20.6%), K. pneumoniae 93 isolates (18.2%), methicillin sensitive S.aureus 72 isolates (14.1%), E.coli 51 isolates (10%), A.baumannii 32 isolates (6.2%) and S.typhi 17 isolates (3.3%). 323 different bacterial strains (63%) were isolated from patients with burn infection who stayed in hospital for 15 days (Table 1). Out of total 513 bacterial isolates, 122 (23.8%) were isolated as single growth, while 391(76.2%) were isolated as mixed growth (Table 2). According to the results of antimicrobial susceptibility tests, most bacterial isolates were resistant to most antimicrobials with high percentages. Out of the 513 bacterial isolates, only 33 isolates (6.40%) were resistant to imipenem 10µg. The results of antimicrobials susceptibility test and overall resistant of 513 bacterial strains to 14 antimicrobials are shown in Table 3 and Figure 2. Of the total 513 bacterial isolates, 464 isolates (90.4%) were MDR, 20 isolates (14%) were XDR and 17 isolates (3.3%) were PDR (Table 4). Pseudomonas aeruginosa was the most common MDR-bacteria, 130 strains (91.5%), while 4 strains (2.8%) were XDR and 8 strains (5.6%) were PDR. All resistant types of all bacterial isolates are shown in Table 4. According to MDDST, Pseudomonas aeruginosa was the most common ESBL-producing bacteria 51 isolates (35.9%) (Figure 3) followed by K.pneumoniae 21 isolates (2.6%), while, no strain of S.typhi was ESBL. All ESBL- producing gram negative bacteria are shown in Figure 4.
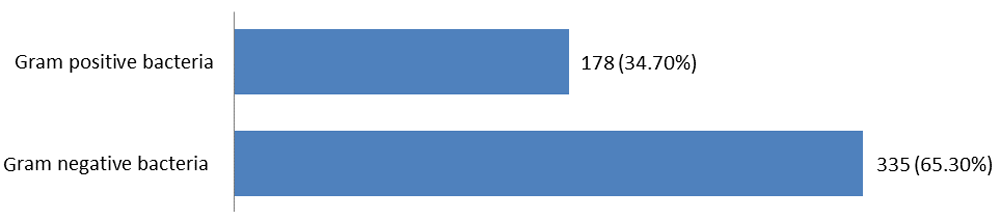
N=513.
N=513.
N=513.
Data were presented as numbers (No.) and percentages (100%) of pathogenic bacteria that were resistant to antimicrobials. AB: Antimicrobials, P: Penicillin 10IU, AX: amoxicillin 25µg, AMC: Amoxiclav 30µg, CRO: Ceftriaxone 30µg, CTX: Cefotaxime 30µg, CAZ: Ceftazidime 30µg, GM: Gentamicin 10µg, TM: Tobramycin 10µg, NA: Amikacin 30µg, CIP: Ciprofloxacin 5µg, IMP: Imipenem 10µg, S: Streptomycin 10µg, E: Erythromycin 30µg, TE: Tetracycline 30µg, MRSA: Methicillin resistance S.aureus, MSSA: Methicillin sensitive S.aureus.
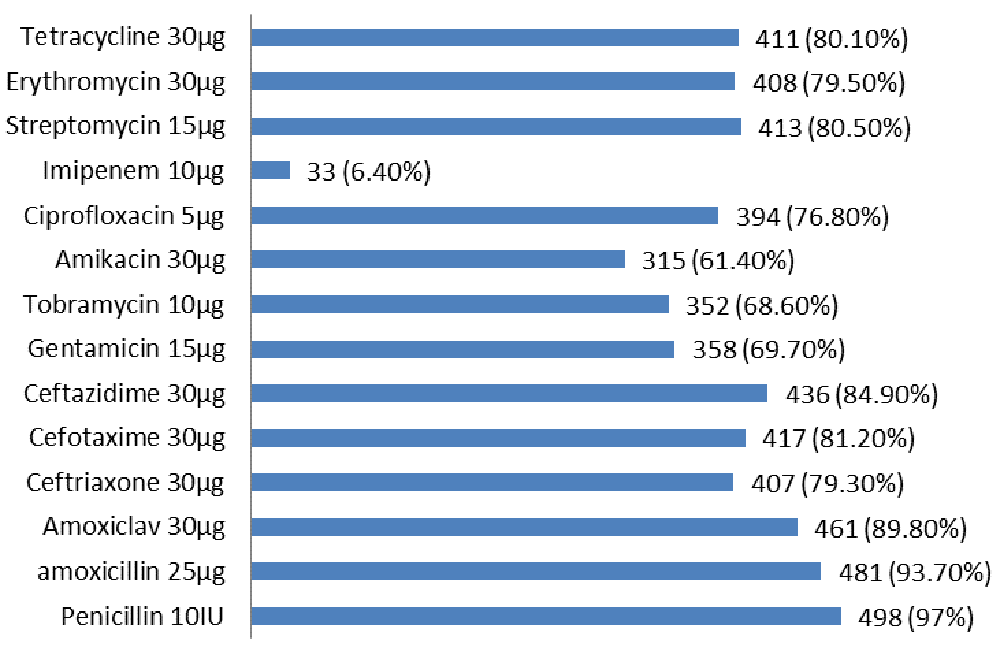
N=513.
N=513.
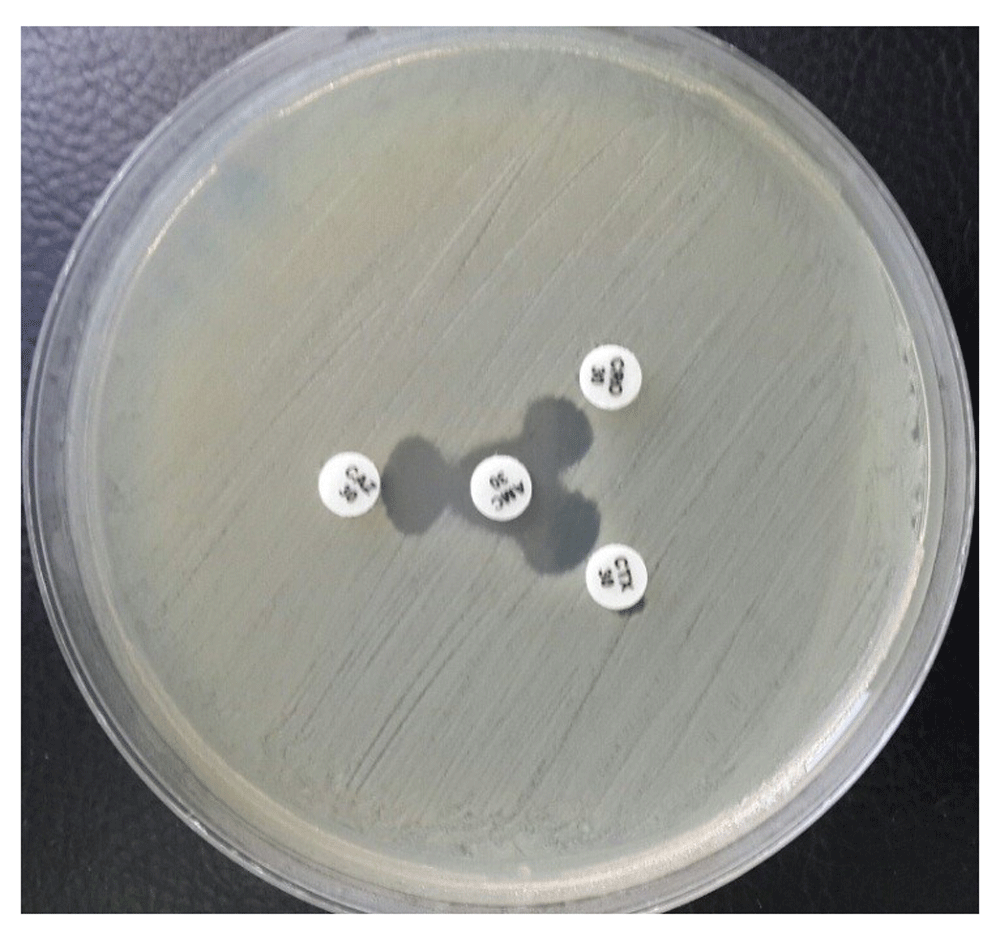
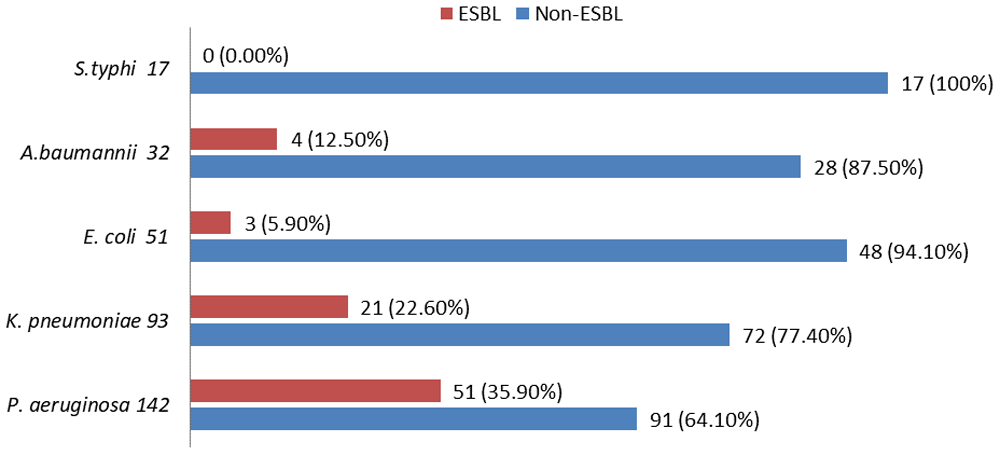
ESBL: extended spectrum beta-lactamase-producing bacteria.
Burn infection is one of the most serious problems in hospitals caused by different pathogens that infect most patients who stay in hospitals for prolonged periods. In this study, 513 different bacterial strains were isolated from 295 swabs of hospitalized patients with burn infections over the three years. Gram negative bacteria were responsible for more than half of infections while gram positive bacteria accounted for 34.7% of overall bacterial isolates. Pseudomonas aeruginosa was the most common bacteria, accounting for 27.6% of total isolates. These results are in agreement with previous studies20–23. Pseudomonas aeruginosa is one of the most important pathogens causing different infections such as bacteremia and burn infections24. This pathogen is well adapted to the hospital environments due to biofilm formation that provides long survival advantages for the pathogen, and effectively prevent eradication by the host immune system or antimicrobial drug treatment25. Pseudomonas aeruginosa has become responsible for more than 70% of mortality in burn patients26,27 . The results of this study showed that MRSA was the second most common bacteria isolated from patients with burn infection, 106 isolates (20.6%) of total bacterial isolates, while, MSSA was the most 4th common pathogen with 72 isolates (14.1%). These results are similar with many previous studies28,29. Staphylococcus aureus is the most common bacteria causing both hospital and community associated infections, including bacteremia, pneumonia and burn infection30. Hospital-associated MRSA accounts for a high proportion of hospitalized infected with S.aureus31. As compare with different bacteria that cause burn infection in hospitalized patients, MRSA-infections is associated with higher morbidity and mortality32. Klebsiella pneumoniae was the most 3rd common bacteria isolated from burn infection in this study with, 93 isolates (18.2%). This result is similar to past studies33,34. Klebsiella pneumoniae is an opportunistic pathogen which causes serious infections like, urinary tract infection, pneumonia, burn infection, and soft tissue infections in compromised and hospitalized patients. It has number of virulence factors such as a capsule that enable this pathogen to colonize and provides phagocytosis resistance35,36. The results of this study showed that Escherichia coli, A.baumannii and S.typhi were prevalent in different percentages, 10%, 6.2% 3.3%, respectively. Our results are similar with some previous studies37–39. On the other hand, the results of the current study proved that there was a positive relationship between a longer stay in hospital and the high prevalence of pathogenic bacteria causing burn infections. Contaminated burning wards and duration of patients stay in hospital, in addition to the size of surface area of burned skin are the most important reasons to increase of persistent and multiplication of pathogenic bacteria in the burned areas40. There are a number of factors that influence the emergence of infection in burns patient including; prolonged hospital stays, contamination of burns wards, nature of burn injury itself, as well as intensive diagnostic and therapeutic procedures41,42. Some studies suggested that burn infection is the most common type of infection, while, others studies reports show it to be bacteremia and pneumonia43,44. In this study, most pathogenic bacteria isolated from burn infection were highly resistant to most antimicrobials, especially against beta-lactams and 3rd generation cephalosporins., All pathogenic bacteria were MDR with high percentages and most of them were XDR,. P. aeruginosa was the most common PDR- bacteria followed by MRSA and A.baumannii. These results are similar with many previous studies5,45–47. Biofilm formation by microorganisms is one of the most important mechanisms in antimicrobials resistant, consisting of the irreversible assemblage of bacterial cells associated with a surface and enclosed in matrix of polysaccharides material48. Biofilms are regarding as a major factor contribution to many chronic inflammatory diseases such as burn infection due to enabling bacteria to colonize the burned skin, altering growth rate and allowing genes to be transcribed that provide these pathogens to high resistance to antimicrobials and host immune system. The overuse and unsuitability of different antimicrobials to treat burn infections has led to the emergence of new MDR, XDR and PDR-bacterial strains that are able to resistant a wide range of many antimicrobials such as aminoglycosides, beta-lactams, cephalosporins, streptomycin and tetracycline49,50. Burn infection in hospitalized patients caused by MDR, XDR and PDR-gram negative and gram positive bacteria such as; P. aeruginosa, K. pneumoniae, MRSA, MSSA and A.baumannii may lead to delays in burn healing, graft lose, as well as development of sepsis and death; therefore, determination of the risk factors for these pathogens infections is essential for infection control47. The results of this study showed that P. aeruginosa and K. pneumoniae were the most common ESBL-producing gram negative bacteria followed by A.baumannii and E.coli while there was no any strain of ESBL- S.typhi. These results are similar to previous studies1,51,52. Infections caused by ESBL-producing gram negative bacteria are associated with an increase of health care costs, morbidity and mortality53,54. Extended spectrum beta-lactamases (ESBLs) have been reported as one of the most important hospital-acquired infections such as burn infection and bacteremia9,55. Most bacteria harboring ESBLs are usually resistant to beta-lactam antibiotics and other classes of antimicrobials56. These enzymes are carried in and transferred from bacteria to bacteria by plasmids57,58. The most important steps to ensure the safety of patients with burn infections are: to control the spread of ESBL-producing bacteria, isolation of colonized patients in sterile wards, and continuously performing antimicrobial sensitivity tests59.
There was a high incidence of MDR-bacteria causing burn infections in Al-Najaf hospital in Al-Najaf City, Iraq. Pseudomonas aeruginosa was the most common MDR, XDR and PDR-bacteria, and the most common of ESBL-producing bacteria causing burn infection over three years followed by MRSA. Imipenem 10µg had good antibacterial activity against more than 93% of bacterial isolates. There was positive correlation between a long stay in hospital and high prevalence of pathogenic bacteria causing burn infection.
In this study, some gram negative and gram positive bacterial isolates are excluded because of the small number of isolates (less than seven isolates over the three years) such as proteus spp (5 isolates), citrobacter spp (4 isolates), enterobacter spp (6 isolates) and enterococcus spp (5 isolates). We think this small number of bacterial isolates don’t has any significant effect on the results of this study.
Dataset 1: Test results for patient swabs 10.5256/f1000research.15088.d21154160
The authors are very thankful to all staff of laboratory of Al-Najaf central hospital in Al-Najaf City for provided all swabs during three years.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Toxicology, microbiology, metagenomics, bioinformatics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomedical waste disposal, hospital infection control, bacteriology, molecular biology, disinfectants.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Jul 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)