Keywords
Ventriculopertioneal shunt infection, Hydrocephalus, children, Staphylococcus aureus, Coagulase-negative staphylococci, Cerebrospinal fluid shunt
Ventriculopertioneal shunt infection, Hydrocephalus, children, Staphylococcus aureus, Coagulase-negative staphylococci, Cerebrospinal fluid shunt
Hydrocephalus is a common and serious neurosurgical condition. The incidence is estimated between 0.4-4 cases per 1000 births worldwide1,2. In some cases there is a treatable underlying cause of hydrocephalus (e.g. a posterior fossa tumour) but in most cases cerebrospinal fluid (CSF) diversion is required. CSF diversion usually requires some kind of shunt, although endoscopic third ventriculostomy is an option in certain types of obstructive hydrocephalus. Different types of shunts are available, of which ventriculoperitoneal (VP) shunts are most frequently used. Other types of shunts include; ventriculoatrial (VA), ventriculopleural and lumboperitoneal (LP)2–5.
Shunt insertion procedures are not without risk and infection is a frequent complication6,7. Rates of infection per shunt vary widely, between 3 and 13%3,6,8–12. Overall shunt infection rate in the UK is estimated at 5.2%7. Shunt infections have a mortality of 10.1% and worse Glasgow Outcome Scale scores, as well as worse school performance in the long-term13. From a public health perspective, shunt infections are associated with major hospital costs, with one episode costing approximately £29,00014. Therefore, prevention or prompt recognition and treatment of infection are imperative.
There are recognised factors known to increase the risk of shunt infection. In general, younger and premature children have a higher risk due to immaturity of their immune system and differences in skin flora compared to older children15. Premature infants who have had shunt surgery before 40 weeks gestational age have the greatest risk of all ages (Hazard radio (HR): 4.72, 95% CI 1.71-13.06)9,10,14,16. Children who have multiple shunt revision procedures, either because of shunt blockage, malfunction, fracture or infection are also at greater risk as with each revision, the cumulative risk rises11. There are technical aspects of shunt surgery that increase risk, including handling of the shunt system with sterile surgical gloves contaminated early in the procedure (HR 1.07, 95% CI 1.02-1.12, p=0.01). The risk is higher still if there is a post-operative CSF leak through the wound, allowing skin flora to access the shunt system (HR 19.16, 95% CI 6.96-52.91, P<0.0001)4,16.
Recognition of shunt infections in children can be difficult as they present with a wide variety of non-specific symptoms such as fever, vomiting, drowsiness, headaches, irritability, seizures and abdominal pain3,10,17. Up to 90% of shunt infections occur within the first three to nine months after shunt insertion. During this period there should be a low threshold for investigating patients who display any of these symptoms3,4,7,12.
The organisms most frequently identified are skin flora, with staphylococcal species accounting for up to 80% of shunt infections9,14,18. Staphylococcus epidermidis is the most frequent coagulase-negative staphylococci (CoNS)7,12. Other common organisms are Staphylococcus aureus (5-26%) and other CoNS (36.1-53%)9–11,17,19. Gram-negative organisms are also frequent findings, accounting for up to 54% of infections in some series20. Gram-negative rods were identified in approximately 7–9% of infections11,17.
Treatment of shunt infection requires complete removal of the device in combination with temporary drainage, unless infection is confined to the abdomen in which case there is a period of shunt externalisation before replacement18,20. Early surgical intervention is especially important since staphylococci commonly form a biofilm after colonising an implant, making it more difficult for antibiotics to penetrate and eradicate infection21. Additionally, early and prolonged intravenous and/or intrathecal antibiotic therapy are needed to sterilise the CSF. It is recommended to wait until the cultures have been sterile for at least 72 hours before implanting a new shunt5. There is some evidence that in case of low-grade inflammation the patient can sometimes be treated with antibiotics alone, but success rates are reported to be low3,4,13,14.
Optimal duration and choice of antibiotic treatment are unclear in the current literature. There is no correlation between recurrence of infection and the duration of antibiotic therapy given, suggesting that a shorter treatment course could be as effective as a longer one22. Due to the increasing concern of multidrug-resistant bacteria, the use of penicillin has largely been replaced by vancomycin18. Linezolid is a good alternative, effective against most Gram-positive microorganisms, including multidrug-resistant strains23. There are currently no randomized studies, large case series, recent prospective studies or guidelines regarding the treatment of ventricular shunt infections in children14.
Given the severity of this condition and the lack of evidence-based management strategies, there is a need for evaluation of current practice to establish a standard of care. Therefore, this study aimed to retrospectively analyse the management of ventricular shunt infections in children in the regional referral centre for the North East of England, the Great North Children’s Hospital in Newcastle upon Tyne, UK. We aimed to describe the clinical presentation, diagnostic efficacy, as well as surgical and medical treatment regimens including antibiotic use. In addition, we hope to identify areas for improvement in clinical practice to reduce mortality, re-infection rates or neurological sequelae in these children.
All children (≤18 years) diagnosed with shunt infections presenting to the Great North Children’s Hospital, Newcastle Upon Tyne, UK over the last 15 years were included in this study. Only children with a permanent shunt device were included. This could either be a VP shunt, a VA shunt, a ventriculopleural shunt or a LP shunt. Patients who had the infection before the year 2000 were excluded from the study, as were patients who were over 18 years old at the time of infection. A shunt infection was defined as any patient coded as ‘shunt infection’ at discharge or any patient who received treatment for suspected shunt infection, with or without microbiological confirmation.
Patient lists were obtained from clinical coding department of the hospital. Using broad search terms ‘shunt infection’, ‘CSF infection’ and patients aged ≤18 years old, 708 possible patients were identified. After screening for eligibility with a digital archive for discharge and referral letters, using the criteria listed above, 65 patients were included in the study (Figure 1).
A proforma (Supplementary File 1) was compiled including all relevant clinical, microbiological and radiological information. It was completed by medical staff using clinical records, laboratory (biochemistry and microbiology) results, radiology scans and reports, discharge and referral letters. Data was analysed using Microsoft Excel 2010. Statistical methods used were non-parametric.
Prior to this study, there was no standard protocol at this institution for the management of CSF shunt infections. It was common practice to give a single dose of cefuroxime at induction in theatre as prophylaxis prior to surgery for shunt insertion. Additionally, gentamicin was used occasionally to flush through the shunt system prior to implantation, depending upon surgeon preference. Antibiotics were not routinely given post-operatively for a first time shunt insertion without suspected infection. Shunt infections were managed on a case-by-case basis. Usually the shunt was removed completely and a new shunt inserted as soon as possible after CSF sterilisation. For the purposes of this study, CSF sterilisation was defined as the first negative CSF culture that remained negative in repeated cultures.
Ethical approval was not required as this was a quality improvement study. The Caldicott principles, a framework of good practice in the use of patient information, were adhered to throughout the study in agreement with local hospital policies. The Clinical Governance Department of the Trust provided Caldicott approval under ID no. 4251. All data were collected and stored securely on password protected hospital computers and all data were anonymised.
A total of 65 patients were included in this study, 55.4% were male (n=36, p=0.19), 46.1% were preterm, 33.8% were born at term and 20.0% unknown due to lack of clinical information. Three different types of shunt were used, VP shunts (n=61, 95.3%) were the most frequently used, two patients had LP shunts and one a VA shunt. Only one shunt was known to be antibiotic impregnated. The majority (n=49, 83.0%) had their first shunt before the age of one year. The median age at which patients received their first shunt was two months (IQR 1-6 months) (Table 1).
Of the 92 episodes, diagnosis relied on a positive CSF culture in 61 (66%) cases, a positive shunt culture in 8 (9%) cases and a positive wound culture in 3 (3%) cases. A further 8 (9%) episodes had negative cultures, but were diagnosed by white blood cell count in the CSF >5/mm3. In the rest of the episodes (n=12, 13%) diagnosis was made solely on clinical data.
The median number of leukocytes in CSF was 46/mm3. A blood culture was obtained in 75% (n=68) of episodes, of which only 3% (n=2) had a positive result. In 43% (n=39) of episodes a CT scan was performed to exclude shunt blockage. In only 5% (n=2) of these episodes the scan was suggestive of infection. Ventriculitis may be seen as irregular enhancement of the ependymal lining of the ventricles. For three of the scans no final report was available.
Of the 65 patients, 42 (64.6%) received prophylactic antibiotics prior to first shunt insertion. The other 23 patients either did not receive antibiotics or had incomplete or missing documentation. Of the patients who received a single dose of antibiotics (n=35), the majority received prophylactic cefuroxime (n=23, 65.7%). Other antibiotics included; vancomycin (n=4. 11.4%), flucloxacillin (n=4, 11.4%), cefotaxime (n=2, 5.7and benzylpenicillin (n=2, 5.7%).
The median age at the first infection was 36 months (IQR 4-100 months) and 40.6% (n=26) had their first infection in the first year of life. Patients presented with a variety of non-specific symptoms. Of all the episodes of infection for which information was available (n=91), 61 (67.0%) presented with fever. Other common symptoms were irritability (n=27, 29.7%), vomiting (n=26, 28.6%), cutaneous manifestations, for example rashes or erythema over the shunt tract, (n=21, 23.1%) and abdominal pain (n=17, 18.7%). Infections with S. aureus presented more often with cutaneous manifestations (n=5, 14%), headache (n=3, 9%) and diarrhoea, (n=3, 9%), while CoNS infections presented more often with irritability (n=13, 18%) (Figure 2).
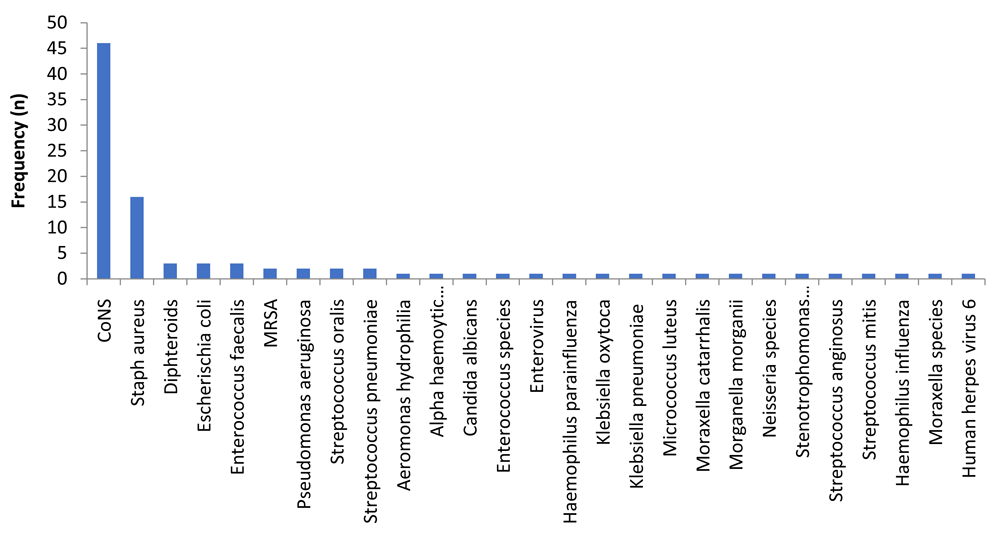
Information regarding treatment regimens was available for 91 episodes. A total of 97 microorganisms could be identified in 73 (80.2%) of the episodes, with 31 different microorganisms causing shunt infection (Figure 3). The two largest groups were human skin flora, CoNS (n=46, 47.4%) and S. aureus (n=16, 16.5%). In 19.8% (n=18) of episodes no microorganisms were identified. Eighty-eight patients (96.7%) received antibiotic treatment. Complete information about the treatment course could be collected from 62 episodes from 45 patients. The median number of days these patients received antibiotics per episode was 18 days (IQR 14-25 days). The shortest period was one day and the longest 52 days. Most patients received more than one type of antibiotic during their treatment course. Only 4.8% (n=3) of episodes were treated with one antibiotic. The median number of different antibiotics received per episode was three, with the maximum of nine in one patient (Table 2).
In total 25 different types of antibiotics were used to treat 88 episodes. Vancomycin was used most frequently, in 58.0% (n=51) of episodes. Furthermore, linezolid was used in 48.9% (n=43), cefotaxime in 43.2% (n=38), meropenem in 38.6% (n=34) and rifampicin in 33.0% (n=29). Other antibiotics were used in less than 20% of the episodes (Figure 4). A schematic representation of the antibiotic treatment per patient was generated, which showed no overall trends in antibiotic use. Table 3 and Table 4 show the different combinations of antibiotics used for five days or more in S. aureus and CoNS infections respectively to give a clearer view of overall use. Empirical use of antibiotics at the start of treatment is highlighted for comparison. We have limited this information to CoNS and S. aureus infections due to a wide variation in other pathogens (n= 1-2 maximum).

| Vanc | Line | Rifa | Fluc | Mero | Cotr | Cefo | Cefu | Metr | Teic | Ceft | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | x | x | |||||||||
| 2 | x | x | x | x | |||||||
| 3 | x | x | x | ||||||||
| 4 | x | x | x | x | x | ||||||
| 5 | x | x | x | ||||||||
| 6 | x | x | x | ||||||||
| 7 | x | x | |||||||||
| 8 | x | x | |||||||||
| 9 | x | x | x | ||||||||
| 10 | x |
X are antibiotics used for ≥5 days overall, highlighted are antibiotics were started empirically at diagnosis.
Vanc = vancomycin, Line = linezolid, Rifa = rifampicin, Fluc = flucloxacillin, Mero = meropenem, Cotr = co-trimoxazole, Cefo = cefotaxime, Cefu = cefuroxime, Metr =metronidazole, Teic = teicoplanin, Ceft =ceftriaxone
X are antibiotics used for ≥5 days overall, highlighted are antibiotics were started empirically at diagnosis.
Vanc = vancomycin, Line = linezolid, Rifa = rifampicin, Fluc = flucloxacillin, Mero = meropenem, Cotr = co-trimoxazole, Cefo = cefotaxime, Cefu = cefuroxime, Cipr = ciprofloxacin, Metr = metronidazole, Teic = teicoplanin, Ceft = ceftriaxone, Cefa = cefalexin, Cefta = ceftazidime, Gent = gentamicin
Regarding surgical management, 67.0% (n=61) had shunt removal, 15.4% (n=14) had shunt externalisation and 26.4% (n=24) were treated without any surgical intervention. Date of sterilisation of the CSF was available for 57 episodes. The time between infection of the shunt and sterilisation of the CSF varied and was between 1 and 28 days. The median was 4 days (IQR 2-10 days). The median number of days between sterilisation of the CSF and insertion of a new shunt was 11 days (IQR 5-17 days).
Recurrent infections occurred in 18/64 patients (28.1%). In seven of these episodes the reinfection was with the same microorganism within 90 days of the previous infection. All seven patients were treated with appropriate antibiotics for the first infection and six of them had sterilised CSF after treatment. Three of the patients had no surgery, one had a shunt externalisation and three had shunt removal for this episode of infection.
Among these 92 infections in 65 patients, a total of 146 new shunts were implanted and a total of 178 shunts were revised, of which 120 revisions were not due to infection, but predominantly due to shunt blockage. Most of the infections took place shortly after insertion or revision of the shunt. 58.8% (n=47) took place within 100 days after insertion of a shunt and 70.4% (n=57) within 100 days of last contact with the shunt (either placement or revision) (Figure 5). 67.5% (n=54) of infections took place within nine months after placement of a new shunt and 58.8% (n=47) within three months. 74.1% (n=60) of the infections took place within nine months of last contact with the shunt and 70.4% (n=57) within three months. There was a significant positive correlation between the number of infections and shunts, Spearman rho 0.247 (p=0.05) and a non-significant correlation between the number of infections and revisions, Spearman rho 0.153 (p=0.23).
Infants and children born prematurely are known to have a higher risk of shunt infection9,10,14,16. In our population 46% of the patients were born prematurely and 41% had the first infection before the age of one year. This reaffirms that in these groups the suspicion of a shunt infection should be promptly investigated and treated. Gender does not seem to be a risk factor for developing a shunt infection11,13,16 and there was no difference between the number of males and females infected (55% male, p=0.19) in our study. In our population 68% of infections occurred within nine months of shunt insertion and 59% within three months. This suggests that the shorter the period after shunt insertion, the higher the suspicion of an infection. Our percentages are lower than those reported in the literature; 90% within three to nine months4,7,12, which may suggest fewer episodes might be attributed to microbial colonisation during surgery in our cohort. It has been shown that the cumulative risk of infection increases with every revision11. Our data also show a significant correlation between number of shunts and number of infections. However, we found no correlation between the number of revisions and the number of infections. This could be because the definition of ‘revision’ is complex and used for a large range of surgical procedures in our records.
Preventative strategies during surgery may influence the incidence of shunt infections. One of the most important actions shown to reduce shunt infections is perioperative prophylactic antibiotics, with the literature supporting their use in all patients undergoing a shunt insertion2,24. A systematic review and meta-analysis by Ratilal et al. demonstrated that antibiotic prophylaxis can significantly decrease the rate of shunt infection (OR 0.51, 95% CI 0.36-0.73)25. Only 65% of the patients in our populations had documented evidence of receiving antibiotics before their first shunt insertion. However, due to incomplete availability of records and a lack of documentation this number is very likely underestimated. Clearer documentation will be necessary in the future to be able to accurately determine this modifiable factor. In the Great North Children’s Hospital it is standard practice to use cefuroxime as prophylaxis before surgery. Cefuroxime covers for infections with S. aureus, but does not cover for infections with CoNS, which was the most frequently found organism causing shunt infection. It may therefore be beneficial to consider using teicoplanin as prophylaxis which is effective against both based on local resistance patterns.
Many different, non-specific symptoms can occur as a consequence of shunt infection3,10,17. In this study, fever was the most common symptom (67% of the episodes), with cutaneous manifestations more frequent with S. aureus infections and irritability associated with CoNS infections. In children with a shunt who complain of fever, irritability, vomiting, cutaneous manifestations or abdominal pain the suspicion of an infection should be high.
S. epidermidis, S. aureus and other CoNS were the most frequently observed and together accounted for 54% of the organisms found. This correlated with findings from other groups from the United Kingdom, the United States of America, Taiwan, Korea, Spain and Germany7,9–12,14,17–20. Another relatively frequent organism in the literature was Propionibacterium acnes19,26,27 which was not identified in our cohort possibly due to the fact this organism is mainly found in patients over the age of 14–15 years old, whereas 88% (n=80) of the episodes in this study occurred before 14 years.
In this cohort, 67% underwent shunt removal and 15% underwent shunt externalisation (n=14, of which 9 went on to have shunt removal). In CSF infections, it is best practice to remove the infected device completely and insert an EVD until the CSF is sterilised18 ,20. However, in infections very soon after insertion of a shunt, treatment with antibiotic alone could be justified, as the microorganisms have not had the time yet to form a biofilm, but there is a paucity of evidence for this at present and currently the safest option is to remove the infected device completely. There are no specific guidelines, but it is considered safe to insert a new shunt when the CSF has been sterile for 48–72 hours5. Since antibiotic treatment should be started as soon as possible, it may be beneficial to develop protocols for the use of empirical antibiotic therapy after CSF sampling at presentation. The agent chosen should cover for at least CoNS and S. aureus, since they were the most common findings. If there is abdominal pain, it may be advisable to choose an agent that also covers for Gram-negative bacteria. This agent should also be able to penetrate the CSF. A combination of linezolid and ceftriaxone would fulfil these requirements, with meropenem as a second line agent. However, specific local resistance patterns should be taken into account. Antibiotics can be rationalised once the CSF culture results are known. The optimal duration of treatment is still unclear,but should be at least seven to twelve days after the last positive culture, and depending on the microorganisms identified, according to expert opinion. It is best to start treatment IV, as this will allow CSF delivery of antibiotic. Once a patient with confirmed ventriculitis has an EVD, antibiotics should be switched to intrathecal administration when possible, since this seems to be the optimal method of antibiotic delivery to CSF28,29.
Limitations of this study included, the incomplete availability of clinical records and incomplete documentation, resulting in information that was not possible to acquire retrospectively, in particular antibiotic prophylaxis, treatment courses and surgical notes. We were unable to obtain the total number of CSF shunts inserted during this period and were therefore unable to calculate the incidence of shunt infections in our region.
Dataset 1: Data outlining the demographics and treatment course for each anonymised patient. 10.5256/f1000research.15514.d21161230
The results presented here have previously been presented as a part of an abstract for the 2016 34th Annual European Society for Paediatric Infectious Diseases meeting and can be found here.
Special thanks to the clinical records department of the Royal Victoria Infirmary for their help in obtaining clinical records.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biofilm infections associated with implants and devices
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 30 Jul 18 |
read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)