Keywords
extremity soft tissue sarcoma, disease-free survival, overall survival, chemotherapy, outcomes
extremity soft tissue sarcoma, disease-free survival, overall survival, chemotherapy, outcomes
Soft tissue sarcoma is a rare tumor that represents less than 1% of all malignancies1. Complete surgical resection with adequate margin is the mainstay of treatment for patients with this condition. The prognosis of patients with soft tissue sarcoma varies according to histologic subtype, tumor size, tumor location, margin status, and tumor depth2–6. Given the heterogeneity of soft tissue sarcomas, it is difficult to determine the value of prognostic factors and the survival outcomes among the different histological subtypes. In term of adjuvant treatment, the impact of adjuvant chemotherapy treatment on survival in soft tissue sarcoma has been investigated in several phase 3 randomized controlled trials7–10 Although those studies found adjuvant chemotherapy effective for improving outcome in soft tissue sarcoma, the efficacy of this adjuvant treatment continues to be debated. Data from meta-analyses and large randomized trials reported by the European sarcoma group indicated postoperative chemotherapy improved relapse-free survival (RFS) in patients with extremity soft tissue sarcoma, but data regarding improvement in OS were conflicting7–14
The aim of this study was to investigate the clinical characteristics, prognostic factors, survival outcomes, and outcomes of treatment in extremity soft tissue sarcoma patients who underwent complete tumor resection at Siriraj Hospital.
Medical records of patients diagnosed with extremity soft tissue sarcoma and treated with total tumor resection at Siriraj Hospital (Bangkok, Thailand) during the January 2007 to December 2016 study period were included. Siriraj Hospital is Thailand’s largest university-based national tertiary referral center. The protocol for this study was approved by the Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (approval no. 835/2559).
Patients aged ≥15 years who were diagnosed with non-metastatic extremity soft tissue sarcoma with tumor >5 cm or World Health Organization tumor grade 3 of any size15 were eligible for inclusion. Patients meeting any one of the following criteria were excluded: distant metastasis prior to resection date, soft tissue sarcoma at non-extremity site and Ewing sarcoma. Collected data included age, gender, ECOG16, tumor size, histological subtype, site, type of surgery, margin status, and postsurgical intervention. Chemotherapy agent/regimen, dose, cycle, and treatment compliance data were also collected in patients who received adjuvant chemotherapy. Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events version 3.0. Regarding survival analysis, disease-free survival (DFS) was defined as the duration from curative surgery to the documented date of relapse. Overall survival (OS) was defined as the duration from diagnosis to either i) the date of death from any cause registered in the Civil Registration Database; or, ii) the censor date of December 31, 2016.
Patient characteristics were described using descriptive statistics. Categorical variables are reported as frequency and percentage, and continuous variables are presented as median and range. Kaplan-Meier survival analysis was performed to estimate DFS and OS. The log-rank test was used for univariate analysis to evaluate the effect of baseline characteristics on survival. The Cox proportional hazard model was used for multivariate analysis. All statistical analyses were performed using SPSS Statistics version 21.0 (SPSS, Inc., Chicago, IL, USA). p<0.05 was regarded as being statistically significant.
A total of 58 patients (25 males, 33 females) with a median age of 53.5 years were included. The histologic subtypes were undifferentiated pleomorphic sarcoma (UPS) (26/58, 44.8%), liposarcoma (8/58, 13.8%), synovial sarcoma (5/58, 8.6%), and malignant peripheral nerve sheath tumor (5/58, 8.6%). In total, 50 of 58 patients had an ECOG score of 0-1, but 8 patients in the non-chemotherapy group had ECOG of 2 or more. Overall median tumor size was 9 cm. Median tumor size was also 9 cm for both the chemotherapy and non-chemotherapy groups. Patient demographic and clinical characteristics are given in Table 1.
A total of 68% of patients received adjuvant treatment after curative resection (81%, 47/58, were limb-sparing surgery and 19%, 11/58 were amputation). Adjuvant radiation therapy was given to 67% (37/58) of all patients. Among the 13 patients in the adjuvant chemotherapy group, 69% (9/13) were treated with doxorubicin and ifosfamide. Other regimens included cisplatin plus doxorubicin in two patients with UPS (in whom it could not be identified whether from bone or soft tissue in origin), and in one patient with leiomyosarcoma (for whom the rationale for this suboptimal usage), and cyclophosphamine–doxorubicin–vincristine regimen in one patient with rhabdomyosarcoma (Table 2). Primary prophylaxis by filgrastim was not routinely administered to patients in this study.
A total of 92% (12/13) of patients in the adjuvant chemotherapy group completed the planned 4 or 6 cycles of chemotherapy. One of 13 patients received only 1 cycle of chemotherapy and was lost to follow-up. Median dose of doxorubicin and ifosfamide was 56 mg/m2 (range: 37.5–73) and 4.8 g/m2 (range: 3.6–5.3), respectively. The reason for chemotherapy dose reduction in most cases was hematological toxicity. A total of 53% (7/13) of patients in the chemotherapy group required dose reduction, and 30% (4/13) needed to delay chemotherapy.
From 69 cycles of adjuvant chemotherapy, the incidence of treatment-related adverse events of any grade was 93% (66/69). No treatment-related death was observed in this study. A total of 23% (16/69) of patients developed grade 3–4 hematologic toxicity. Grade 3 mucositis developed in one patient. Febrile neutropenia occurred in two patients (15.4%). In total, two patients experienced non-hematologic toxicities that led to dose reduction or treatment discontinuation. No cardiac toxicity was reported in patients who received doxorubicin (Table 3).
At a median follow-up of 51 months, 46.7% (21/45) and 30.87% (4/13) of patients in the non-chemotherapy group and chemotherapy group had disease recurrence, respectively (p=0.38). Distant metastasis occurred in 80% (20/25) of all relapse patients. The most common site of metastasis was the lung (15/20) (Table 4). Median DFS in all patients was 33 months. Median DFS in the chemotherapy group was 56.2 months versus 20.5 months in the non-chemotherapy group (p=0.29) (Figure 1). Regarding OS, only 20% of patients had died by the censor date of December 21, 2016. The median OS in the entire 58 patient study population was 74.8 months, with a cumulative survival at 4 years of 84%. Median OS in the chemotherapy group was slightly shorter than in the non-chemotherapy group (66.6 vs 77.2 months, respectively; p=0.24) (Figure 2). Cumulative survival at 4 years in the chemotherapy group was 92% vs 81% in the non-chemotherapy group.
| Site of relapse | Chemotherapy, n | No chemotherapy, n | All, n (%) |
|---|---|---|---|
| Total | 8 | 37 | 45 |
| Local | 0 | 5 | 5 (20) |
| Distant | 4 | 16 | 20(80) |
| Metastatic site | |||
| Lung | 4 | 11 | 15(60) |
| Bone | 0 | 3 | 3 (12) |
| Lymph node | 0 | 2 | 2 (8) |
The treatments given after disease relapse are shown in Table 5. Among the 25 patients with disease relapse, 18 (72%) of them proceeded to post-relapse interventions. There were three patients with distant recurrence that exhibited no evidence of disease (NED) after subsequent treatment. Of these three patients, one underwent pulmonary resection, and the other two achieved complete remission after receiving palliative chemotherapy.
| Treatment | Chemotherapy, n | No chemotherapy, n | Total, n (%) |
|---|---|---|---|
| Total | 4 | 21 | 25 (100) |
| Any treatment | 2 | 16 | 18 (72) |
| Radiation | 0 | 2 | 2 (8) |
| Surgery | 1 | 6 | 7 (28) |
| Systemic therapy | 1 | 8 | 9 (36) |
Kaplan-Meier survival analysis was performed to estimate DFS and OS, the log-rank test was used for univariate analysis to evaluate the effect of baseline characteristics on survival, and the Cox proportional hazard model was used for multivariate analysis (Table 6). Shorter duration from curative surgery to first date of chemotherapy administration was identified as a significant favorable prognostic factor for DFS (p=0.02). Median duration from surgery to first chemotherapy administration was close to being significantly shorter in the non-relapse group than in the relapse group (28 vs 45.5 days; p=0.059). Tumor grade 3 with size >10 cm was a baseline factor found to be a significant predictor of unfavorable DFS (p<0.001); median DFS in this group was 9.4 months versus 36.4 months in the others. No other independent factors, including histologic subtype, age, margin status, ECOG, or type of surgery, were significantly associated with DFS. In subgroup analysis of patients who received chemotherapy, no significant difference in DFS was observed relative to any baseline characteristics. Tumor grade 3 with size >10 cm showed a trend toward worse OS, but the association did not achieve statistical significance (hazard ratio: 2.8, 95% CI: 0.8-9.9; p=0.09) (Figure 3). No clinical characteristics were found to be significantly correlated with either DFS or OS in multivariate analysis.
| Clinical characteristics | HR | 95% CI | p-value |
|---|---|---|---|
| Positive margin | 1.41 | 0.52-3.78 | 0.50 |
| ECOG performance status ≥2 | 0.89 | 0.26-3.10 | 0.85 |
| Post-surgical intervention | 0.55 | 0.23-1.31 | 0.18 |
| Histological grade 3 | 1.35 | 0.57-3.2 | 0.48 |
| Tumor size >10 cm | 1.56 | 0.70-3.50 | 0.27 |
| Tumor size >10 cm (grade 3) | 4.7 | 2.07-10.74 | <0.001* |
| Shorter duration from diagnosis to surgery** | 0.02 |
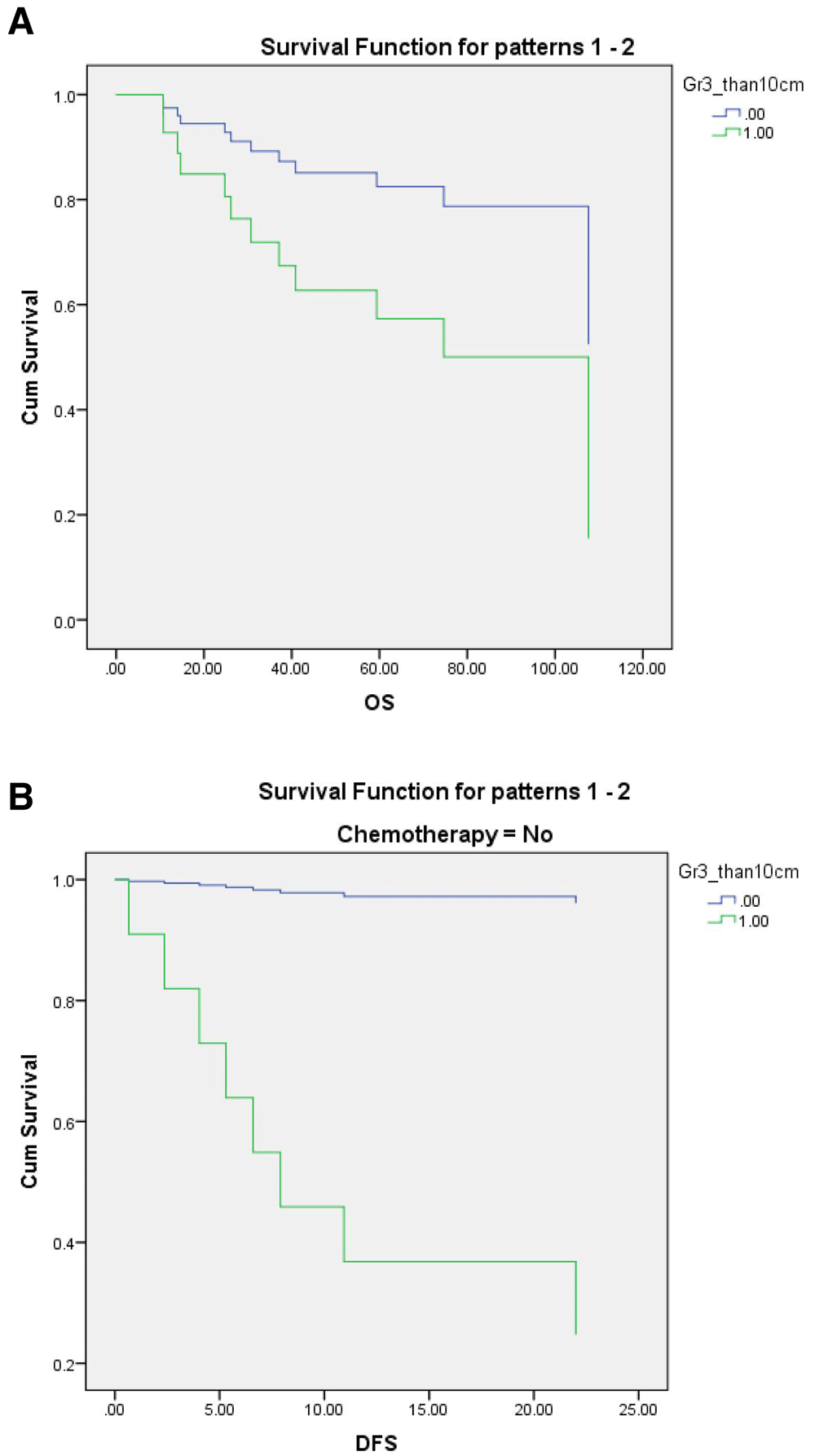
Kaplan-Meier curves of overall survival (OS) (A) and disease-free survival (DFS) (B), separated tumor grade 3/>10 cm size status.
The aim of this study was to investigate the clinical characteristics, prognostic factors, survival outcomes, and outcomes of treatment in extremity soft tissue sarcoma patients who underwent complete tumor. We included only patients with high-risk extremity soft tissue sarcoma in order to minimize the heterogeneity of the study population.
Regarding baseline characteristics, the median age of 53.5 years in our study was much higher than the median age of 46 years reported in the European Organization for Research and Treatment of Cancer (EORTC) and Italian Sarcoma Group (ISG) trials9,13, but similar to a study by Liu et al. from Taiwan18. The median tumor size of 9 cm found in this study was slightly smaller than the median tumor size of 10 cm reported from the ISG trial9. However, the median tumor size of 9 cm in both the chemotherapy and non-chemotherapy groups in this study were larger than the median tumor size of 8.6 cm and 7.5 cm in the chemotherapy and non-chemotherapy groups in the EORTC trial, respectively12. The predominant histological subtype in this study was UPS (26/58, 45%), which is higher than the 29% and 18% rates reported from the ISG trial9, EORTC trial13, respectively, but similar to 27% rate reported by Roger et al.19 Synovial sarcoma in this study was 8.9% (5/58), which is considerably lower than the 25% rate in the ISG and the 14% rate in the EORTC trial9,13. A total of 87% (50/58) of patients had negative surgical margin, which was higher than 37%, 38% and 37% reported in the EORTC, Taiwan and Korean studies, respectively13,18,20.
For tumor grading, subjects in our study had more tumor grade 1 than patients in the ISG trial (7/58; 12% vs 0%)9. In our analysis, we also attempted to identify baseline characteristics that portend an unfavorable prognosis. In previous studies, tumor size and grading were reported to be prognostic markers, especially if the tumor was larger than 10 cm or larger than 5 cm and of a high grade2–6. This study revealed that tumor grade 3 >10 cm was associated with significantly shorter DFS. Shorter duration from curative surgery to commencement of chemotherapy administration was found to be a significant favorable prognostic factor for DFS (p=0.02).
Moreover, the median time from surgery to first chemotherapy administration in non-relapse patients was nearly significantly shorter than in relapse patients (28 vs. 45.5 days; p=0.059). This 4-week period is comparable to the reported 4-week time frame within which chemotherapy was commenced in several previous studies7–14. We found tumor grade 3 with size >10 cm showed a non-significant trend toward association with worse OS.
From our survival analysis, the median follow-up period was 51 months, which is similar to durations of follow-up reported in previous studies7–14,18–20. Overall median DFS was 33 months in our study population. Median DFS in this study was 56 months in the chemotherapy group and 20.5 months in the non-chemotherapy group, which are longer than the 48-month and 16-month DFS durations, respectively, reported in the ISG trial9. One possible explanation for this difference between studies is that our study consisted of patients with lower risk of tumor grade 3 and size >5 cm for relapse, as compared with 100% of patients in the ISG trial9. In this study, 8.6% (5/58) of patients had tumors smaller than 5 cm, and 12% (6/50) had tumor grade 1. Only 24% (12/50) of patients in our study had tumor grade 3 that was larger than 10 cm, which is much lower than the 54% reported from the ISG trial9.
When we compared the median DFS in this study with the 90-month and 78-month DFS of EORTC study in the chemotherapy and non-chemotherapy groups, respectively13, the shorter survival in our study was probably due to greater heterogeneity among patients in the EORTC study. This heterogeneity included 27% with small tumors, less cases with tumor grade 3 (46% in EORTC vs. 30/50; 60% in our study), various primary sites, and median tumor size (7.5 cm and 8.6 cm in the chemotherapy and non-chemotherapy groups, respectively) that was smaller than the median tumor size by group in our study. The 4-year cumulative OS in the chemotherapy and non-chemotherapy groups in this study was 92% and 81%, respectively, compared to 69% and 50%, respectively, in the ISG trial9.
The efficacy of adjuvant chemotherapy was also evaluated. We proceeded with our analysis despite potential limitations, which included those inherent to retrospective studies, and unbalanced baseline characteristics and sample sizes between the chemotherapy and non-chemotherapy groups, which could result in some degree of selection bias relative to several clinical characteristics. The median age of patients in the chemotherapy group was lower than that in the non-chemotherapy group (42 vs 55 years, respectively). Moreover, chemotherapy group patients had an ECOG performance status score of 0-1, while all ECOG 2-3 patients were put into the non-chemotherapy group. As such, both age and ECOG score may influence physician decision. A total of 13 patients (22%) in our study received adjuvant chemotherapy compared to 34% reported in Taiwan, which had similar baseline patient characteristics to our study18. On the contrary, a report from Korea demonstrated only 7.3% of patients received adjuvant chemotherapy20.
For survival analysis compared between patients who received and who did not receive adjuvant chemotherapy, we found no statistically significant difference for either DFS or OS between groups (56 vs 20.5 months, respectively; p=0.29). It should be noted that this study was a retrospective review. There are, therefore, many confounding factors involving the benefits of chemotherapy in this small sample size of patients that received chemotherapy (13/58; 22%). Secondly, chemotherapy may not improve survival benefit, even in high-risk population as established in previous studies, including a long-term (5-year) follow-up evaluated by an Italian Sarcoma Group study and a Cochrane Review of Sarcoma Meta-analysis Collaboration10,11,13.
The incidence of distant relapse in this study was 34.4% (20/58), as compared to the relapse rate of 47% (49/104) in patients studied in the ISG trial9. The 4-year cumulative incidence of distant metastasis in ISG study in the chemotherapy and non-chemotherapy groups was 44% and 45%, respectively, compared to 31% and 37% in our study. Post-relapse treatment was also evaluated. In this study, 18/25 (72%) patients were commenced on interventions that included surgery, radiation, and/or chemotherapy.
Compliance with adjuvant chemotherapy was also evaluated and reported. Most patients were given doxorubicin and ifosfamide, except in specific subtypes (Table 2). The median dose of doxorubicin and ifosfamide was 56 mg/m2/cycle and 4.8 g/m2/cycle, respectively, which was lower than reported doses from previous studies7–14. We found that 53% (7/13) of patients needed dose reduction or delay in treatment due to hematologic toxicity. Most cases did not use prophylactic growth factor to prevent febrile neutropenia. This may explain the higher rate of dose reduction in our study compared to the dose reduction of 26% of patients in the ISG study9. All patients in the ISG trial received growth factor for secondary prophylaxis. Non-fatal non-hematologic toxicity was found in 62% (43/69) of patients in this study, which was similar to rates reported in previous studies7–14.
This study has some notable limitations. As previously described, the potential for selection bias, incomplete medical records, and disproportionate sample size between the chemotherapy and non-chemotherapy groups made it difficult to evaluate contributing factors. The patients enrolled in this study were also from a single center, which is located in a major urban metropolis. Our findings may, therefore, not be generalizable to other geographic settings within Thailand. Moreover, our center is Thailand’s largest tertiary referral hospital, which means that we are often referred patients with complicated and intransigent. Lastly, tumors with heterogeneous histology subtype with a wide range of intrinsic tumor behavior, such as mitotic rate, grade, and undetermined molecular subtype were included. Further study is warranted in a more specific population.
Tumor grade 3 >10 cm was the only baseline clinical characteristic found to be a significant predictor of unfavorable DFS in univariate analysis. No conclusive benefit of adjuvant chemotherapy was observed relative to DFS and OS.
Dataset 1. Complete raw demographic and clinical information for each patient included in this study. DOI: https://doi.org/10.5256/f1000research.15793.d21294717.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Sarcomas
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 03 Aug 18 |
read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)