Keywords
Sangre de Drago, Croton lechleri Muller Arg., Croton urucurana Baill, antimicrobial activity
Sangre de Drago, Croton lechleri Muller Arg., Croton urucurana Baill, antimicrobial activity
In Ecuador, there is a growing interest in medical plants from medical practitioners1,2. “Sangre de Drago“, the name by which the plant and its medicinal latex are known, are a group of Croton (Euphorbiaceae) tree species widely distributed in Latin America. In Ecuador, they are found in the Amazon region and, as in Bolivia, Colombia and Peru, the most commonly identified species of Sangre de Drago is Croton lechleri Muller Arg3. Croton urucurana Baill, a species found in the subtropics of South America, which is representative of the north of the country, islands and coasts of the Uruguay River, does not appear in the literature information for the Croton genus in Ecuador4.
The red latex and bark of Sangre de Drago have been used in popular South American indigenous medicine for different purposes, including wound healing5, diarrhoea, influenza, tonsillitis, intestinal disorders, herpes, fertility enhancement, tuberculosis6, hepatitis, cancer prevention, anti-inflammatory problems, acne, weight loss, coughs and colds7. In Ecuador, C. lechleri is used for treating haemorrhages, wounds and tuberculosis2. The chemical composition of latex has been widely studied, revealing the presence of different metabolites, amongst which are the alkaloid taspine and its salt, lignans, proanthocyanidins, terpenes and phenolic compounds. These metabolites are responsible for the different activities that this medicinal plant presents. Taspine and lignans have anti-inflammatory activity, taspine hydrochloride has wound healing activity, and phenolic compounds have antimicrobial and antiviral activity8. The antimicrobial activity of Sangre de Drago in Gram-positive bacteria has been demonstrated, such as Staphylococcus aureus ATCC 6538 and Staphylococcus epidermidis ATCC 12228; and with the Gram-negative Pseudomonas and Klebsiela FDA 6029. In this study, the antimicrobial activity of latex and of alcoholic extracts of leaves and bark of two species of Sangre de Drago was evaluated in vitro against specific bacterial strains in order to compare their antibacterial properties.
The vegetal material used from the two species of Sangre de Drago was collected in the localities of Talag and Canelos belonging to the provinces of Napo and Pastaza, respectively, and located at 1°, 3’, 57” south latitude; 77°, 54’, 26” west longitude; 40° west longitude; and 1°, 35’, 22” south latitude; 77°, 44’, 48” west longitude; 40° west longitude, respectively. The sample collection was permitted by the Ministerio del Medio Ambiente, Ecuador (MAE), (registration number: 2015-RO. Nro. 449 and 905-RO.- suplemento 553). Aerial parts (leaves and bark) and latex of the trees aged 3 to 4 years and in a flowering-fructification stage were used. The crude latex for the analysis was obtained from indigenous merchants in the areas. One of the two latex samples corresponding to the Canelos sector was extracted from the same tree from which the plant material was collected. The collecting folders for each species were prepared and sent to the National Herbarium of Ecuador (QCNE) for taxonomic identification. The folder regarding the material collected in the Province of Napo was identified as C. lechleri Muller Arg. and the one corresponding to the province of Pastaza as C. urucurana Baill. The aerial parts (leaves and bark) of the two species were dried at 49°C, in a JP SELECTA series 0385081 stove with circulating air for 3 days. The plant material was removed at least once a day for uniform drying until a constant weight was achieved. Next, the parts were crushed in a hammer mill until a moderately thick powder was obtained. The batches of the plant material were identified for the corresponding analyses with the letters “l” and “b” for leaves and bark, respectively, accompanied by the letters T and C to identify provenance from Talag and Canelos, respectively. The latex samples were refrigerated at 4°C for 2 weeks, during which time their analysis began from their acquisition. They were coded with the letters “T” and “C” and the letter “C” was accompanied with numbers “1” and “2” for the latex provided by the indigenous merchants and for the latex obtained from the same tree as the vegetal material was collected, respectively.
The extraction of the alcoholic extracts (20 % Tincture) of the leaves, bark and latex from the two Croton species was carried out by maceration using 70% alcohol as a menstruum, at room temperature, for 2 to 7 days, with shaking at least twice a day. A 20% tincture was obtained, from which the physical and chemical parameters were determined as indicated by the Ecuadorian Quality Control Standard for natural medicinal products. Under the same conditions, the physical-chemical constants of the crude latex of Sangre de Drago samples were determined. For the determination of the refractive index, a Clean-Prison series 003021 ABBE refractometer was used. pH determination was carried out in a Metrohm potentiometer, type 1.744.0010.
The fractionation of the analysed alcoholic extracts was achieved by thin-layer chromatography, tapping 0.1 ml of the alcoholic extract by means of a glass capillary 1 cm from the bottom edge of 60GF254 silica gel plates. It was allowed to dry for a moment before each application and at the end of it. Then the plates were placed in saturated glass chambers for at least 30 minutes with steam from the components of each of the mobile phases used, which are listed below as ratios:
• Butanol-acetic acid-water (BAW): 4-1-5
• Chloroform-acetone-diethylamine: 7-3-1
• Toluene-ethyl acetate: 70–30
The following were used as developers: 254 nm and 366 nm ultraviolet light, Dragendorff reagent, ammonia fumes. Each chromatography experiment was repeated twice.
The microbiological quality of the alcoholic extracts of leaves and barks, as with crude latex, was verified as outlined by the World Health Organization (WHO)10. The tally of bacteria, fungi and yeast was read after 5 days of incubation as indicated by the WHO. Regarding the reading for the determination of pathogenic bacteria, this was performed at 24 and 48 h of incubation with two repeats. The results of bacterial growth were compared with that described by Porter and Kaplan11 for each culture medium used, for the purpose of bacterial recognition.
The evaluation of the antimicrobial activity was carried out against six bacterial strains (Escherichia. coli, S. aureus, Pseudomonas aeruginosa, S. epidermidis, Bacillus subtilis and Streptococcus sp.) maintained in nutritious agar at 4°C according to Garcia and Uruburu12. The antifungal activity was not assessed since growth problems were encountered for the strain Candida albicans ATCC10231. The method used was described in the “CYTED” Research Techniques Manual9. The extracts were solubilized using dimethylsulfoxide (DMSO) to their respective dilutions (5000, 2500, 1250, 1000, 500, 250 and 125 p.p.m.). Gentamicin sulfate (0.1 mg/0.1 ml) was used as a standard antibiotic and as a growth inhibition solution. As a negative control, Muller Hilton Agar and Muller Hilton Agar boxes with DMSO (0.1 ml) were prepared.
Table 1 shows the results of density, total solids, pH and refractive index of the alcoholic extracts of the bark, leaves and latex of the two Sangre de Drago varieties analyzed.
As can be seen, there is no marked difference between the values of density, total solids and pH of the different extracts analysed. The organoleptic characteristics, on the other hand, differ clearly between the extracts of the two Croton species. In the same way, the capillary analysis shows a clear differentiation between the extracts and basically between the latex extracts (Figure 1). The presence of a fluorescent blue colour on the fringe of the capillary analysis image was characteristic in all the analysed extracts. When the wet paper strip was exposed to ammonia fumes, revealed with 366 nm UV light and the environment left to dry, a weaker blue color was observed on the paper strips with 366 nm UV light. According to the consulted literature13, these results are characteristic of flavonoid-anthocyanidin type metabolites.

Table 2 shows the values of the physical and chemical parameters of the crude latex of the two varieties of Sangre de Drago analysed. As can be seen, the organoleptic characteristics allow the two varieties to be clearly differentiated. In terms of density parameters, total solids and pH, the results vary for each species. The refractive index cannot be determined by the physical characteristics of the samples (colour and viscous consistency).
Figure 2 presents the results of the thin layer chromatography, RF of the spots and colours visualized with the developers: 366 nm UV light, 254 nm UV light, ammonia fumes, Drangendorf reagent, alcoholic extracts of leaves, bark and latex.
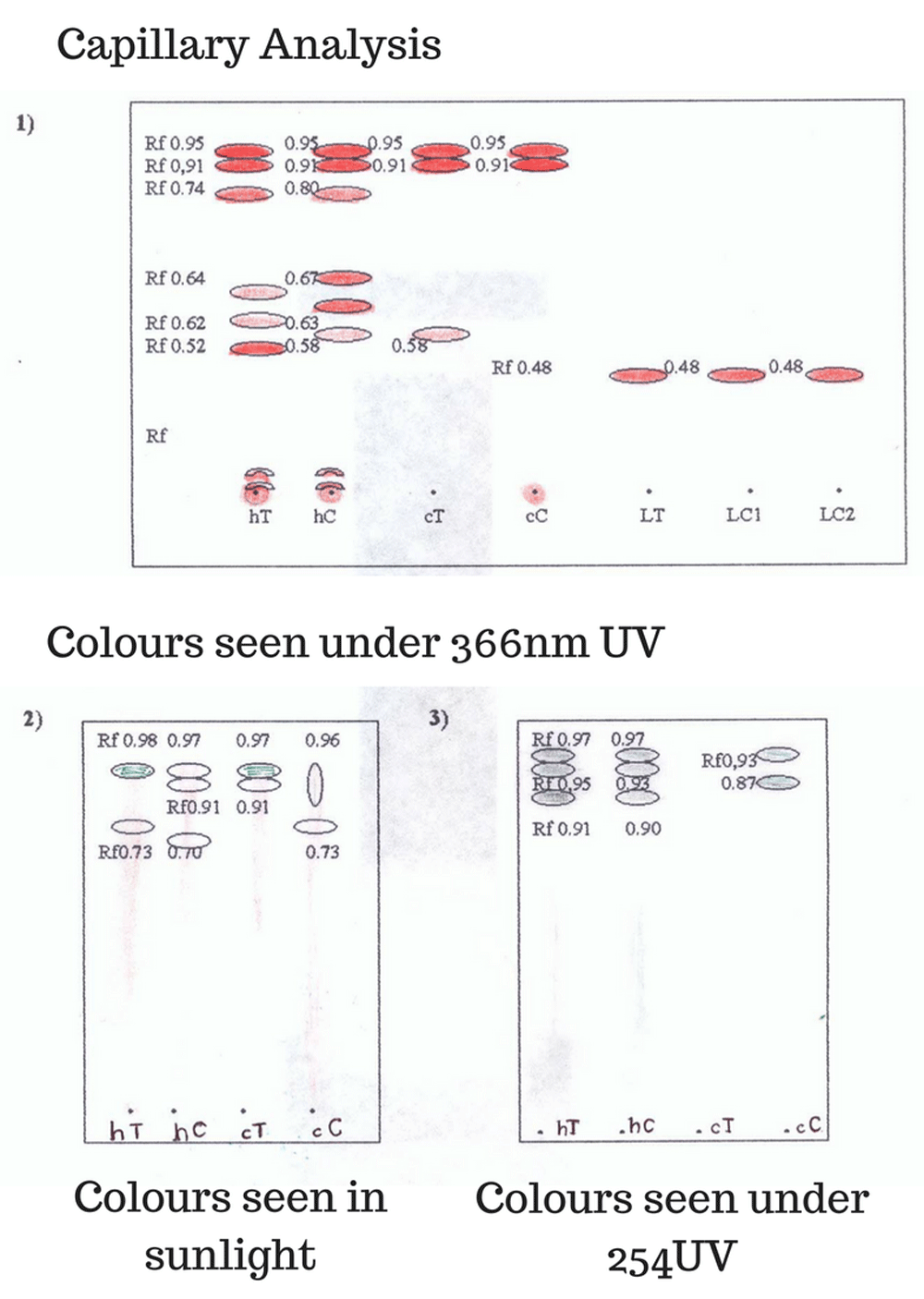
As can be seen, the separation of the respective fractions occurs with greater clarity in the plates run in the mobile phases of BAW and 70–30 toluene-ethyl acetate. The different fractions obtained are observed as yellow at the point of application and faint orange to the naked eye during the course of the shift.
When revealed with 366 nm UV light, fluorescent orange spots are observed at different RF. When revealed with 254 nm UV light, faint grey spots are observed. The plates were passed through ammonia fumes and observed under 366nm UV light, where it was observed that the orange-coloured dots turned fluorescent blue. The phases were run in chloroform-acetone-diethylamine (7-2-1) mobile phase. Under natural light, no stain can be seen, but under the 366nm UV light, reddish spots are observed at a 0.48 RF and under 254 nm UV light, blue spots are observed. The RF of the calculated spots is closer to that reported in the literature for the alkaloid taspine (Theoretical RF of 0.50). When revealing the spots with Drangendorf’s reagent, a faint brown color was observed that disappeared after a few seconds. The presence of orange spots that turn blue in the presence of ammonia is characteristic of flavonoid-type phenolic compounds, and within these the anthocyanidins group, which are components present in Sangre de Drago14.
The results obtained from the count of bacteria, fungi and yeasts of the alcoholic extracts of leaves, bark and crude latex in colony forming units/ml of sample are found in Table 3.
As can be observed, the values corresponding to the microbiological count of bacteria of the alcoholic extracts are within those established for crude drugs (see Table 4). The other two samples of latex did not present bacterial growth. The count of moulds and yeasts presents similar results to the bacterial count in the alcoholic extracts. The same cannot be said for the crude latex, because on the fifth day, the three analysed samples presented massive growth, which did not allow for the counting of the colony forming units. The presence of pathogenic batteries was negative for the alcoholic extracts and for the latex samples from “C1“ Talag and Canelos. This was not so for the “C2“ Canelos latex sample, which showed growth of pink colonies, characteristic of E. coli in red Violet Agar. The abundant bacterial growth with a metallic luster in Mac Conkey Agar of the planting in lines of characteristic colonies in Red Violet Agar confirmed the presence of E. coli. It was impossible to count the colony forming units, as they surpassed what was established by the WHO for this type of sample. All samples were negative for bacterial growth in Cetrimide Agar specific for P. aeruginosa and in Baird Parker Agar for S. aureus.
Source: Quality Control Methods for Medicinal Plant Materials9
For the determination of the antibacterial activity by means of the method described in the CYTE Research Techniques Manual9, a battery of six bacteria was used (E. coli, S. aureus, P. aeruginosa, S. epidermidis, B. subtilis and Streptococcus sp.). The preliminary results of each of the bioassays performed are presented in Table 5, in which the moderate antimicrobial activity (±) for B. subtilis can be observed in the seven samples analysed (extracts and crude latex). For the remaining five bacteria, the antibacterial activity was negative (-) for the alcoholic extracts. The Sangre de Drago latex coded “T“ and “C1“ were active (+) for S. epidermidis at doses of 2500 p.p.m. and 5000 p.p.m. and the “C2“ latex is active (+) at a dose of 1250 p.p.m. For the four remaining bacteria, they did not exhibit antimicrobial activity. The negative activity (-) for E. coli does not agree with previous reports15–17. Regarding B. subtilis, it could be said that it is similar to results from other authors’ studies. The literature does not present information relating to activity on S. epidermidis. As for fungal activity, this test was not performed due to growth problems that arose with the C. albicans strain.
1. The results of the physical-chemical constants of the alcoholic extracts do not allow one to differentiate the two Croton species studied. However, the organoleptic characteristics and the capillary analysis allow for differentiation between the alcoholic extracts and latex of C. lechleri and C. urucurana.
2. The organoleptic characteristics of the latex of the two varieties allow one to differentiate them from each other. Regarding physical-chemical parameters, the values are very similar, which allows them to be used for their differentiation.
3. The average pH value, of 3.7 for C. lechleri latex, 4.6 for C. urucuran C1 latex and 3.9 for C. urucurana C2 latex, are similar to that found in the literature (pH 4.4).
4. The chromatographic analysis of the extracts of bark, leaves and latex of the two Sangre de Drago species allowed for the separation of this species’ characteristic components, alkaloids and phenols. These compounds make up the metabolites responsible for the biological properties of the Sangre de Drago.
5. The antimicrobial activity of the alcoholic extracts of leaves, bark and latex was negative for S. epidermidis and E. coli, but not for B. subtilis, which was moderately positive.
6. The three latex samples show antimicrobial activity for S. epidermidis. However, the sample of Canelos latex, obtained from the same tree that leaves and bark were collected from, presented antimicrobial activity at lower doses, 1250 p.p.m. Antimicrobial activity was not found in the reviewed literature. In relation to B. subtilis, moderate activity was observed in the three latex samples, an activity that was also evident in the alcoholic extracts of leaves and bark.
7. The values of antimicrobial activity against E. coli and B. subtilis are found in the literature15–17 for C. Lechleri, but not for C. Urucurana, a species for which no information is available.
Dataset 1. Images and raw data from all chromatography experiments under all development methods with RF values, in addition to complete microbiological counts and physical/chemical characteristics of extracts. Also included are images of the plates used in this study, although it should be noted that these images were captured after storage for 2 or more years. Chromatography data 1 contains data generated using a mobile phase of Butanol:acetic acid:water (4:1:5) visualized using ferric chloride. Chromatography data 2 contains data generated using a mobile phase of chloroform:acetone:water (7:3:1) visualized using ammonia vapor; Chromatography data 3 contains data using a mobile phase of chloroform:acetone:diethylamine (7:3:1) visualized using ammonia vapor. DOI: 10.5256/f1000research.14575.d21135818.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbial genetics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Bandara KRV, Padumadasa C, Peiris DC: Potent antibacterial, antioxidant and toxic activities of extracts from Passiflora suberosa L. leaves.PeerJ. 2018; 6: e4804 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Cancer research, diabetes, antimicrobial, signalling pathways etc..
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Aug 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)