Keywords
Leishmaniasis, Leishmania major, Solanum nigrum, Plant extracts, Toxicity
This article is included in the Pathogens gateway.
This article is included in the Neglected Tropical Diseases collection.
Leishmaniasis, Leishmania major, Solanum nigrum, Plant extracts, Toxicity
Leishmaniasis is a widespread disease caused Leishmania parasites which are transmitted by the sandfly. Desjeux (1998) reported that the disease occurred in different clinical forms, which ranged from cutaneous self-healing lesions to a fatal visceralizing form, and also included the metastasizing muco-cutaneous and post-kala-azar dermal leishmaniasis. Leishmaniasis represents an important health and socioeconomic problem in 88 countries around the world, where this disease is endemic according to a study by Dujardin et al. (2008). Despite the death toll and disease burden of leishmaniasis, there is an acute lack of suitable therapies. Treatment of the disease depends on a limited number of drugs with limitations such as high cost, unacceptable host toxicity, poor efficacy, lack of availability, and acquired parasite drug resistance as reported in studies by Barrett & Fairlamb (1999); Fairlamb (2003) and Stuart et al. (2008). Therefore, the development of cheap, available, effective and less toxic drugs is of paramount importance. Medicinal plants are the best alternative since they possess natural active components that can be effective against parasitic infections or can be used in development of commercial drugs.
Several studies have reported that some plants are effective against Leishmania parasites both in vitro and in vivo. The findings of studies by Kinuthia et al. (2014); Ndeti et al. (2016) and Njau et al. (2016) revealed that plants such as Allium sativum, Callistemon citrinus, Moringa stenopetala and Aloe secundiflora were effective against Leishmania major parasites. A study by Wabwoba et al. (2010) indicated that a combination of M. stenopetala with Allium sativum induced apoptotic effect in Leishmania major promastigotes. Similar results were reported in studies by McClure et al. (1996) where the growth of Leishmania mexicana and Leishmania chagasii were inhibited by A. sativum, and by Khademvatan et al. (2011) where A. sativum extract induced apoptosis in Leishmania major parasites. Schlein et al. (2001) reported that Ricinus communis (Malpighiales: Euphorbiaceae) possessed anti-leishmanial effects both in Phlebotomus duboscqi (Diptera: Psychodidae), the vector for L. major in Israel, and in in vivo in BALB/c mice when used alone, as revealed in a study by Oketch et al. (2006), or in combination with Azadiracta indica (Sapindales: Meliaceae) as indicated by Jumba et al. (2015).
The findings of some studies have shown that Solanaceae family plants have medicinal effects against various parasitic infections. The findings of Laban et al. (2015) showed that Solanum aculeastrum was effective against Leishmania major parasites. Additionally, Mishra et al. (2013) reported that a prenyloxy-naphthoquinone obtained from roots of Plumbago zeylanica (Caryophllales: Plumbaginaceae) has anti-leishmanial activity against Leishmania donovani. There was a significant difference between the EC50 for the isolated compound and miltefosine, the standard drug (P< 0.001) against L. donovani promastigotes and amastigotes. Studies on L. major in Kenya by Makwali et al. (2015) have shown that Plumbago capensis possess anti-leishmanial effects. The current study evaluated the in vitro anti-leishmanial activity of Solanum nigrum extracts on L. major parasites.
The proposal for this research work was submitted to the KEMRI Scientific Steering Committee (SSC), for approval and was given ethical clearance (Number: KEMRI/SSC-2028) on the use of the mice as the animal model by the Ethical Review Committee (ERC). All experimental animals at the end of the experiment were sacrificed by injection of 100 µl sodium pentobarbital and disposed of according to the regulations of Animal Care and Use (ACUC) through incineration.
The in vitro studies were carried out using a comparative study design. Pentostam (Glaxo Operations (UK) Limited, Barnard Castle, UK) and amphotericin B (AmBisome®; Gilead, Foster City, CA, USA) were used as the standard drugs to compare their efficacy and toxicity with those of the test extracts. RPMI-1640 and Schneider’s Drosophila media (Thermo Fisher Scientific, Waltham, Massachusetts, USA) were used as the control in in vitro experimental chemotherapeutic studies.
Fresh leaves of Solanum nigrum were collected form Kisii and Bungoma, Kenya, where the plant is abundant. The plants were transferred to the Center of Traditional Medicine and Drug Research (CTMDR) at KEMRI (Nairobi, Kenya) and dried at 25°C until they became brittle and attained a constant weight. The dried plants were separately ground using an electric mill (Christy& Norris Ltd., Chelmford, England) into powder followed by extraction using water and analytical grade methanol. The methanolic extracts were prepared as described by Mekonnen et al. (1999) and Cock (2012). Immediately, 100 g of ground plant material was soaked in 500 ml of analytical grade methanol for 72 h at room temperature with gentle shaking. The mixture was filtered using Whatman No.1 filter papers and concentrated using a rotary evaporator to obtain dry methanolic extracts. The extracts were coded as A and B for methanolic extracts of S. nigrum (Bungoma) and S. nigrum (Kisii), respectively. The aqueous extracts were prepared as described by Delahaye et al. (2009). Briefly, 100 g of the dried ground plant material in 600 ml of distilled water was placed in a water bath at 70°C for 1.5 h. The mixture was filtered using Whatman No.1 filter papers and then the filtrate frozen, dried and weighed. The extracts were coded as C and D for S. nigrum (Kisii) and S. nigrum (Bungoma), respectively. The extracts were then stored at 4°C until required for bioassays.
A total of four 8-week-old male inbred BALB/c mice with weights that ranged between 25 and 29 g were obtained from KEMRI. There were eight BALB/c mice per cage in the animal house kept at 23–25°C under a 12/12 h light/dark cycle and were fed on standard diet in the form of mouse pellets and given tap water ad libitum. The mice were handled in accordance with the regulations set by the Animal Care and Use Committee at KEMRI. The mice were used for extraction of peritoneal macrophages that were used for anti-amastigote assay.
The Leishmania major strain (IDUB/KE/94=NLB-144) which was originally isolated in 1983 from a female Phlebotomus dubosqi collected from Marigat, Baringo County in Kenya were used. The parasites were grown to stationary phase at 25°C in Schneider’s Drosophila medium supplemented with 20% heat-inactivated fetal bovine serum (FBS) (Hyclone® USA), 100 U/ml penicillin and 500 µg/ml streptomycin (Hendricks & Wright, 1979), and 250 µg/ml 5-fluorocytosine arabinoside (Kimber et al., 1981). The stationary-phase metacyclic stage promastigotes were then harvested by centrifugation at 1500g for 15 min at 4°C. The metacyclic promastigotes were then used for the in vitro assays.
Stock solutions of the crude plant extracts were made in Schneider’s Drosophila culture medium for anti-leishmanial assays and filtered through 0.22-µm filter flasks in a laminar flow hood (Biological Safety Cabinet). The stock solutions were then stored at 4°C and retrieved later for both in vitro bioassays.
This assay was used to test the cytotoxicity of individual extracts against Vero cells (Thermo Fischer Scientific, Waltham, Massachusetts, USA) and the results were presented as IC50 values. The assay was carried out as described by Wabwoba et al. (2010). Vero cells were grown in minimum essential medium (MEM) (ATCC® 30-2003™) supplemented with 10% FBS, penicillin (100 IU/ml) and streptomycin (100 µg/ml) in 25 ml cell culture flasks incubated at 37°C in a humidified 5% CO2 for 24 h. The Vero cells were harvested by trypsinization and pooled in 50-ml centrifuge tubes from which 100 µl of cell suspension was moved into two wells of rows A-H in a 96-well flat-bottomed microtiter plate at a concentration of 1×106 cells per ml of the culture medium per well and incubated at 37°C in 5% CO2. The MEM was gently aspirated off and 150 µl of the test extracts (A, B, C and D) were added at concentrations of 1000 µg/ml, 500 µg/ml, 250 µg/ml, 125 µg/ml, 62.5 µg/ml and 31.25 µg/ml in the micro titer plates. The plates containing the Vero cells and test extracts were further incubated at 37°C for 48 h in a humidified 5% CO2 atmosphere. The controls wells comprised of Vero cells and medium while the blank wells had medium alone. A total of 10 µl of (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reagent were added into each well and incubated further for 2–4 h until a purple precipitate (formazan) was visible under the microscope. The media together with MTT reagent was gently be aspirated off, after which 100 µl of dimethyl sulfoxide (DMSO) was added, and vigorously shaken for 5 minutes in order to dissolve formazan. The absorbance (optical density) was measured for each well plate using a microtiter plate reader at wavelength of 570 nm. The IC50 values of the extracts were determined automatically using the Chemosen program 2.
The MICs were determined as described by Wabwoba et al. (2010). Briefly, the L. major metacyclic promastigotes at concentration of 1×106 promastigotes per ml of the culture medium were treated with individual methanolic test extracts A and B whose concentrations were 2000 µg/ml, 1000 µg/ml, 500 µg/ml and 250 µg/ml. These test procedures were repeated for aqueous extracts C and D. The lowest concentration of the individual test extracts in which no live promastigotes were observed was the MIC.
Metacyclic promastigotes at a concentration of 1×106 promastigotes per ml of the culture medium were grown for 48 h in 24-well microtiter plates at 25°C. Aliquots of the promastigotes were transferred into 96 well microtiter plates and incubated further at 27°C for 24 h after which 200 µl of the highest concentrations (2 mg/ml) of the individual test extracts were added before serial dilutions of 2.0×103, 1.0×103, 5.0×102, 2.5×102, 1.25×102, and 6.25×101 were carried out. The control wells contained L. major promastigotes in culture medium alone whereas the blank wells had the culture medium alone. The plates were incubated further at 27°C for 48 h and 10 µl of MTT reagent was into each well and incubated further for 4 h. The medium and MTT reagent were aspirated off the wells. Next, in each well, 100 µl of DMSO was added and the plates shaken for 5 minutes. Absorbances were read at 562 nm using a microtiter reader. The absorbance readings were used to generate IC50 values for the different plant extracts using the Chemosen program v2.
Survival of L. major promastigotes was stratified as follows: ++++, 75–100% survival compared with control; +++, 50–<75% survival compared with control; ++, 25–<50% survival compared with control; +, <25% survival compared with control; -, absence of live promastigotes.
The anti-amastigote assay was carried out as described by Delorenzi et al. (2001). The peritoneal macrophages were obtained from four clean BALB/c mice. The mice were anaesthetized using 100 µl pentobarbital sodium (Sagatal®). The body surface of the mouse was disinfected with 70% ethanol after which it was opened dorso-ventrally to expose the peritoneum. A total of 10 µl sterile cold PBS was injected into the peritoneum. After injection, the peritoneum was gently massaged for 2 min to dislodge and release macrophages into the PBS. The peritoneal macrophages were then be harvested by withdrawing the PBS. The PBS containing the macrophages was washed through centrifugation at 2,000g for 10 min and the pellet obtained was re-suspended in RPMI-1640 culture medium. The macrophages were adsorbed in 24-well plates for 4 h at 37°C in 5% CO2. Non-adherent cells were washed away with cold sterile PBS and the adherent macrophages were incubated overnight in RPMI-1640 culture medium. Adherent macrophages were then infected with L. major promastigotes and were further incubated at 37°C in 5% for 4 h, after which they were washed with sterile PBS to remove the free promastigotes, which were not engulfed by the macrophages. This was followed by incubation of the preparation for 24 h in RPMI-1640 culture medium.
Pentostam and liposomal amphotericin B at concentrations of 125 µg/ml, 250 µg/ml and 500 µg/ml were used as positive control drugs to compare the parasite inhibition with that by plant extracts. The medium and test extracts or drug was replenished daily for 3 days. After 5 days, the macrophages were washed with sterile PBS at 37°C, fixed in methanol and stained with 10% Giemsa. The number of amastigotes was determined by counting at least 100 macrophages in duplicate cultures, and the count was expressed as infection rate (IR) and multiplication index (MI) as described by Berman & Lee (1984) in the calculations below:
IR (%) = Number of infected macrophages per 100 macrophages
The IC50 values were determined using Chemosen program v2. Data for infection rates and multiplication indices were saved as percentages and then were analyzed using SPSS 13.0 programme. The results were expressed as mean values ± standard deviation (SD). Statistical analysis were done using one way ANOVA and Tukey’s post hoc test and p values < 0.05 were considered significant.
Generally, all the test extracts studied were less toxic (i.e. higher IC50 values) to Vero cells when compared to the control drugs, pentostam (0.03 mg/ml) and amphotericin B (0.01 mg/ml) (Table 1).
The initial concentration of the test extracts was 1000 µg/ml while that of control drugs was 500 µg/ml before serial dilution.
The methanolic and aqueous extracts tended to show a dose-dependent cytotoxic effect which corresponded to low IC50 values as their concentration increased. Methanolic extracts of S.nigrum from Bungoma and Kisii had IC50 values of 0.57 mg/ml and 0.50 mg/ml while those their aqueous counterparts were 0.76 mg/ml and 0.64 mg/ml (Table 1).
Preliminary studies involved exposure of the L. major promastigotes to extracts and control drugs at varying concentrations in vitro. The L. major parasites cultured in Schneider’s Drosophila medium were taken as the negative control because the parasites continued to multiply. Both pentostam and amphotericin B used were able to inhibit the Leishmania major promastigotes growth at an MIC of 31.25 µg/ml. The Schneider’s Drosophila medium, on the other hand, led to maximum survival of L. major promastigotes (++++) (Table 2).
Both aqueous extracts of S. nigrum from Bungoma and Kisii (C and D) inhibited the survival of Leishmania major promastigotes at an MIC of 2000 µg/ml. S. nigrum methanolic extracts from Bungoma (A) lowered L. major parasites at a concentration of 1000 µg/ml, while that from Kisii (B) inhibited L. major multiplication at an MIC of 500 µg/ml.
The concentrations of the extracts that were effective against L. major promastigotes in vitro were high (>0.5 mg/ml) compared to those of the standard drugs (0.03125 mg/ml). The efficacy of methanolic extracts was better than their respective aqueous counter parts (Table 2).
The MICs of S. nigrum from Bungoma (A) and S. nigrum from Kisii (B) methanolic extracts were 1000 µg/ml and 500 µg/ml, respectively. Both aqueous extracts of S. nigrum from Kisii (C) and S. nigrum from Bungoma (D) had MIC values of 2000 µg/ml. The standard drugs had lower MICs (31.25 µg/ml) against L. major promastigotes compared to the test extracts. Schneider’s Drosophila medium, which was the negative control, supported maximum survival of Leishmania major promastigotes (Table 3).
The extracts concentration ranged from 2000 µg/ml to 250 µg/ml for MIC and 62.5 µg/ml for IC50 determination, while the concentration of the positive controls ranged from 125 µg/ml to 15.625 µg/ml for MIC and 500 µg/ml to 15.625 µg/ml for IC50 determination.
IC50 values determined indicated the effectiveness of the test extracts or the controls in inhibiting the promastigotes by 50%. Pentostam and amphotericin B had IC50 values of 0.08 mg/ml and 0.04 mg/ml, respectively, while S. nigrum from Bungoma (A) and S. nigrum from Kisii (B) methanolic extracts had IC50 values of 1.81 mg/ml and 1.28 mg/ml, respectively. Similarly, aqueous extracts of S. nigrum from Kisii (C) and S. nigrum from Bungoma (D) had IC50 values of 1.18 mg/ml and 1.84 mg/ml, respectively. Based on the IC50 values, the aqueous extract of S. nigrum from Kisii (C) was the most effective of the extracts (Table 3).
For extracts that shared the same MICs, higher IC50 values tended to correspond to increased in vitro survival of promastigotes of Leishmania. The methanolic extract of S. nigrum from Kisii seemed to be more efficacious in vitro since it knocked out the promastigotes at a lower MIC level (0.5 mg/ml) when compared to all other extracts whose effective MIC level was ≥1 mg/ml (Table 3).
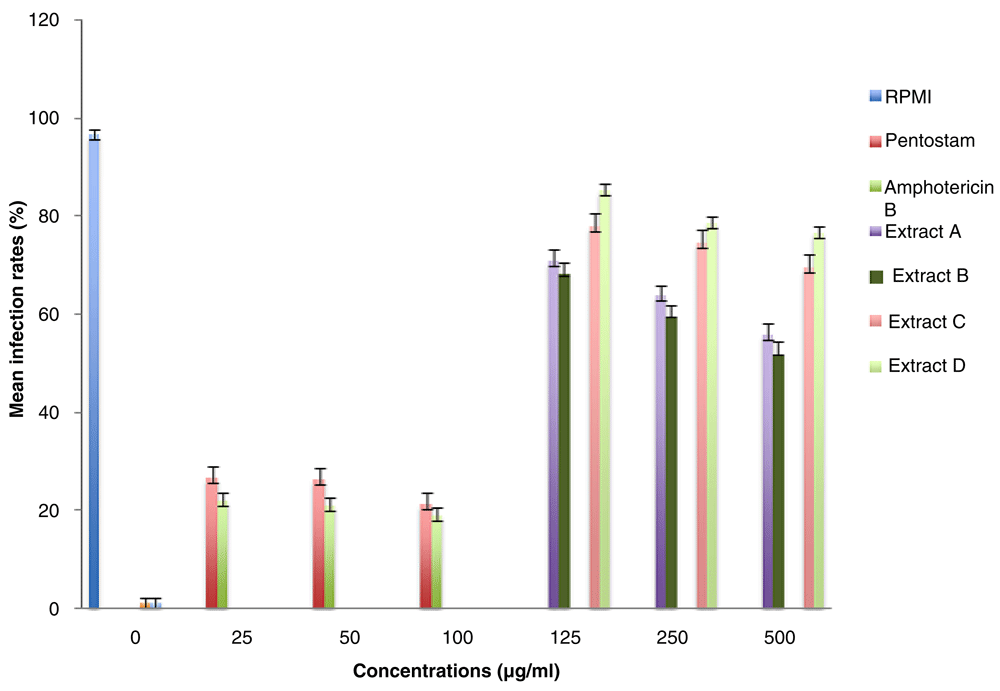
RPMI-1640 medium with no drug had an infection rate of 96.7% (Table 4), which implied that it supported maximum growth of Leishmania major amastigotes in peritoneal macrophages (Figure 1). The leishmaniasis drugs pentostam and liposomal amphotericin B inhibited the in vitro survival of L. major amastigotes, corresponding to low infection rates of 26.3% and 21.0%, respectively, at a concentration of 50 µg/ml (Table 4).
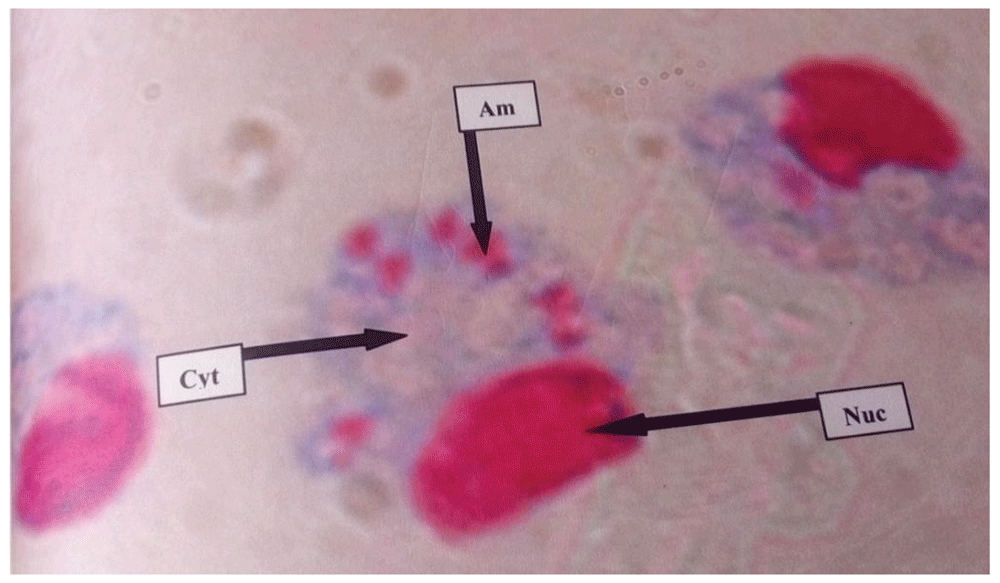
Am= amastigote, Nuc= Nucleus, and Cyt= Cytoplasm.
At a concentration of 125 µg/ml, the methanolic extracts of S. nigrum from Bungoma (A) and S. nigrum from Kisii (B) had infection rates of 71.0±2.3% and 68.0±2.7%, respectively. Similarly, the infection rates of aqueous extracts, S. nigrum from Kisii (C) and S. nigrum from Bungoma (D) were 78.0±2.5% and 85.3±1.2% (Table 4).
The methanolic extract of S. nigrum from Kisii (B) inhibited the survival of L. major amastigotes better than the other extracts in all the concentrations studied (Figure 2). High concentrations of the test extracts and control drugs resulted in low IRs and MIs of L. major amastigotes. The efficacies were dose-dependent. The difference between the IRs of test extracts and the control drugs were statistically significant (P< 0.05).
When the MIs of amastigotes in peritoneal macrophages treated with 125 µg/ml of methanolic test extracts (A and B) were compared with those treated with 50 µg/ml of amphotericin or pentostam, using one-way ANOVA, there was a statistically significant difference (P<0.001). A Tukey post hoc test revealed that the MI of methanolic extracts of S. nigrum from Kisii (A and B) at 500 µg/ml were statistically significant from that of pentostam and amphotericin B (P= 0.001).
When the infection rates of methanolic extracts of 500 µg/ml A and B were compared with those of amphotericin B using Tukey’s post hoc test, the difference in each case was statistically significant, (P<0.001 and P=0.001, respectively). Comparisons of the IRs for extracts C and D with those of amphotericin B or pentostam followed a similar trend, where Tukey’s post hoc test indicated a significant difference (P<0.05) for each comparison. The MIs of pentostam and amphotericin B were not statistically different (P≥ 0.05) at a concentration of 25 µg/ml.
This study has shown that S. nigrum has anti-leishmanial activity against Leishmania parasites. The results indicated that the plant extracts of S. nigrum obtained from Kisii and Bungoma have the potential to inhibit L. major promastigotes in vitro. The current study further established that the concentrations of the extracts that were effective (MIC) against L. major promastigotes in vitro were relatively high (>0.5 mg/ml), as compared to those of pentostam and amphotericin B, which both inhibited the promastigotes at 0.03125 mg/ml. The efficacy of methanolic extracts was better than their respective aqueous counterparts. Schneider’s Drosophila medium was used as a negative control and supported maximum survival of the L. major promastigotes in vitro. This was expected because this medium supports the growth of Leishmania promastigotes and amastigotes, as described by Hendricks & Wright, 1979. The efficacy of test extracts was higher than that of Schneider’s Drosophila medium. The slight differences that have been noted between the two allopatric plants could be due to factors such as difference in the presence and composition of the phytochemicals; a study by Aritho et al. (2017) on T. vogelii also revealed such differences.
A study by Son et al. (2003) showed that extracts from S. nigrum leaves had the potential to be used in treatment of tumors, especially liver cancer, and also used for treatment of lung cancer, bladder and gastric carcinoma as indicated by studies done by Mueller et al. (2005) and Ashwani et al. (2012). Additionally, studies by Jain et al. (2011) and Ashwani et al. (2012) revealed that methanol crude extracts obtained from Solanum nigrum possessed antioxidant activity due to its DPPH radical scavenging activity.
Studies by Estevez et al. (2007); Filho et al. (2013); Hubert et al. (2014); and Shen et al. (2012) revealed that some species of Solanum had antileishmanial activity. Findings from the study by Estevez et al. (2007) showed that the extracts of S. stramonifolium had activity against L.amazonensis amastigotes. This activity was attributed to steroid derivatives which include cilistol A and steroidal alkaloids, which form the main components in Solanum species (Abreu Miranda et al., 2013; Filho et al., 2013).
Cytotoxic assays using Vero cells showed that the test extracts were less toxic compared to the standard antileishmanial drugs. Generally, the increase in the dose of the extracts led to a higher cytotoxic effect on L. major promastigotes, resulting in inhibition of the growth of the parasites. Many drugs used for treatment of leishmniasis are highly toxic (Santos et al., 2008) and this study confirmed that pentostam and amphotericin B are toxic compared with the extracts tested. The continued use of the contemporary leishmaniasis drugs despite their toxicity is mainly due to lack of an alternative. The use of herbal medicine can be a cheaper and available alternative. The aqueous extracts of both S. nigrum from Bungoma and Kisii (IC50, 0.76 mg/ml and 0.64 mg/ml, respectively) were less toxic than methanolic extracts (IC50 of 0.57 mg/ml and 0.50 mg/ml, respectively).
The lower the toxicity of the test extracts, the higher the viability of Vero cells after exposure to extracts and vice versa. According to Das et al. (2007) the Solanaceae family plants have been reported to be poisonous both to humans and livestock. Their toxicity has been attributed to the presence of tropane alkaloids, which when ingested in large quantities, causes anticholinergic effects. Another study by Glossman-Mitnik (2007) reported that the toxicity of S. nigrum which is edible is due to solanine, a glycoalkaloid which causes toxicity as the concentration increases.
The S. nigrum from Bungoma (A) and S. nigrum from Kisii (B) methanolic crude extracts had infection rates of 71.0±2.3% and 68.0±2.6%, respectively, at a concentration of 125 µg/ml. Similarly, the infection rates of aqueous extracts, S. nigrum from Kisii (C) and S. nigrum from Bungoma (D) were 78.0±2.5% and 85.3±1.2%, respectively. In comparison, the leishmaniasis drugs, pentostam and liposomal amphotericin B inhibited the in vitro survival of L. major amastigotes more effectively and this corresponded to low infection rates of 26.3% and 21%, respectively, at a concentration of 50 µg/ml. There was a significant difference between the efficacy of the test extracts and that of the Leishmania drugs (P<0.05). This observation indicates that S. nigrum extracts which are known for their antimicrobial and antifungal potential (Abbas et al., 2014; Musto, 2014). When the test extracts were compared with the controls, IR of macrophages by L. major amastigotes in plain RPMI-1640 medium (negative control) was 96.7±0.9%. This agrees with Berman & Wyler (1980) who observed that the amastigotes of Leishmania tropica and Leishmania donovani in peritoneal macrophages multiplied about three fold in six days when grown in RPMI-1640 medium in absence of antileishmanial agents. The trend was similar for MIs. This observation was similar to that by Wabwoba et al. (2010), who observed that the IRs of amphotericin B and pentostam at 100 µg/ml were 9.0% and 11%, respectively. In this study, however, although the difference between MI for amphotericin B and pentostam at 50 µg/ml was not statistically significant, the in vitro efficacy of amphotericin B in suppressing the amastigotes multiplication was higher than that of pentostam.
The findings of this study have justified the claimed medicinal importance of Solanum nigrum as a remedy for various infections. It can be concluded that the crude extracts of S. nigrum possess considerable anti-leishmanial activity, especially against Leishmania major, which were used in this study. The plant may contain potent anti-parasitic compounds, effective in the treatment of Leishmania infections. However, further investigation needs to be conducted on pure compound isolation, toxicological studies and clinical trials so as to use the promising compounds as effective antileishmanial agents.
Dataset 1. Raw data for absorbance values from MTT assay and subsequent calculation of IC50 values on Vero cells for extracts of Solanum nigrum from Kisii and controls. For sorted raw absorbance data, columns 3, 6, 9 and 12 contain untreated cells; wells A1, A2, A4, A5, A7, A8, A10, A11, B1, B2, B4, B5, B7, B8, B10 and B11 contain medium only. Rows C-H contain indicated test samples, with extract concentrations of 31.25, 62.5, 125 , 250 µg/ml, 500 and 1000 µg/ml, respectively, and control drug concentrations of 16.125, 31.25, 62.5, 125, 250 and 500 µg/ml, respectively. DOI: https://doi.org/10.5256/f1000research.15826.d214921 (Mutoro et al., 2018).
Dataset 2. Raw data for absorbance values from MTT assay and subsequent calculation of IC50 values (on Vero cells) for extracts of Solanum nigrum from Bungoma. For sorted raw absorbance data, columns 3, 6, 9 and 12 contain untreated cells; wells A1, A2, A4, A5, A7, A8, A10, A11, B1, B2, B4, B5, B7, B8, B10 and B11 contain medium only. Rows C-H contain test samples, with extract concentrations of 31.25, 62.5, 125, 250, 500 and 1000 µg/ml, respectively. DOI: https://doi.org/10.5256/f1000research.15826.d214922 (Mutoro et al., 2018).
Dataset 3. Raw data for absorbance values from MTT assay and subsequent calculation of IC50 values (on promastigotes) for extracts of Solanum nigrum from Kisii and controls. For sorted raw absorbance data, columns 3, 6, 9 and 12 contain untreated cells; wells A1, A2, A4, A5, A7, A8, A10, A11, B1, B2, B4, B5, B7, B8, B10 and B11 contain medium only. Rows C-H contain test samples, with extract concentrations of 62.5, 125, 250, 500, 1000 and 2000 µg/ml, respectively C to H for extract samples and 16.125, 31.25, 62.5, 125, 250 and 500 µg/ml, respectively, for standard drugs. DOI: https://doi.org/10.5256/f1000research.15826.d214923 (Mutoro et al., 2018).
Dataset 4. Raw data for absorbance values from MTT assay and subsequent calculation of IC50 values (on promastigotes) for extracts of Solanum nigrum from Bungoma. For sorted raw absorbance data, columns 3, 6, 9 and 12 contain untreated cells; wells A1, A2, A4, A5, A7, A8, A10, A11, B1, B2, B4, B5, B7, B8, B10 and B11 contain medium only. Rows C-H contain test samples, with extract concentrations of 62.5, 125, 250, 500, 1000 and 2000 µg/ml, respectively. DOI: https://doi.org/10.5256/f1000research.15826.d214924 (Mutoro et al., 2018).
Dataset 5. Anti-amastigote (macrophage) assays. DOI: https://doi.org/10.5256/f1000research.15826.d214929 (Mutoro et al., 2018).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. Srivastava S, Mishra J, Gupta A, Singh A, et al.: Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasites & Vectors. 2017; 10 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Dujardin JC, Campino L, Cañavate C, Dedet JP, et al.: Spread of vector-borne diseases and neglect of Leishmaniasis, Europe.Emerg Infect Dis. 2008; 14 (7): 1013-8 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 22 Aug 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)