Keywords
stroke, MCAO, depression, anxiety
stroke, MCAO, depression, anxiety
This newer version of the article has dot plots of the data, rather than bar graphs, to indicate spread. It also has an indicator of the spread of naive data in each graph. Naïve data has been added to the Behaviour.xlsx file in dataset 1. The a priori power calculations used in this study, and post-hoc calculations based on the outcomes of the study, have also been included.
The title has been changed to: An exploratory investigation of ‘depression-like’ behaviours in a model of left-sided distal middle cerebral artery occlusion in young, male C57B6 mice
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Major depression is an important neuropsychiatric consequence of stroke, and develops in approximately one third of stroke patients, often independent of functional deficits1,2. Modelling affective deficits in rodents is key to the development of novel therapeutic strategies, but has proved challenging thus far. Rodent models of post-stroke depression often combine middle cerebral artery occlusion (MCAO) models with models of chronic stress3. Whilst this results in a semi-reproducible set of animals showing both lesions and depression-like behaviours, it is not necessarily representative of the human condition.
The majority of ischemic strokes occur in the territory of the middle cerebral artery4. As such this is most commonly modelled in rodents5. The introduction of the use of endovascular treatment for acute ischemic stroke means that the transient model of stroke has good face and construct validity6. However, model variability still blights pre-clinical stroke research and direct translation of useful therapies has been limited7,8. The permanent MCAO model results in a consistent cortical infarct which shows reproducible evolution. This model is used to mimic human stroke without reperfusion, which represents the majority of clinical strokes.
The evolution of the lesion results in both local central nervous system (CNS) inflammation and changes in systemic immune reactivity9,10. MCAO is known to induce activation of microglia outside the territory of the MCA, even into the contralateral hemisphere11, a finding which translates to human stroke where microglial activation has been found in regions distant from the core infarct12,13. Inflammation within the CNS, both from internal activation of glial cells and from infiltrating immune cells and inflammatory mediators, is a known causative factor in depressive-like behaviours, both in rodents and humans14–16. Indeed in a number of CNS diseases, including stroke, widespread inflammation and depression are now being causally linked17–19. As such, the inflammation associated with the distal MCAO model, which has also been shown to extend into the contralateral hemisphere20 could potentially result in a depression-like phenotype.
Studying affect in rodents provides its own challenges, with the most common models of depression involving either transgenic animals or models of chronic stress21. Combining these approaches with models of stroke results in a rodent model which shows very little face validity, but rather a complex model of vascular disease and stress. In addition, the use of methodologies commonly used to study depression, such as the forced swim test, may be incompatible with animals with limb deficits22 and, as such, hampers our capacity to investigate depression-like behaviours in rodents.
Therefore, the aim of this study was to investigate the effect of stroke on some very basic tests of exploration, motivation and escape behaviours. We used the distal permanent model of MCAO (pMCAO), which gives consistent cortical lesions with minimal surgical trauma, and aimed to study behaviour and lesion volume to determine whether this model induced a depression-like phenotype.
Animals: Adult (8–10 weeks), male C57BL/6/J mice (Taconic Ltd., Ry, Denmark) were housed in individually ventilated cages with a standard sawdust and nesting material mix, and kept under diurnal lightning conditions with ad libitum access to food and water. Animals were housed in groups of 5 and an n of 5 was used per group, a single animal was considered as one unit. Animals were pre-assigned to time and sham/stroke groups (see Animal Numbers, Dataset 123). Experiments were approved by The Danish Animal Inspectorate under the Ministry of Food and Agriculture (permit number: 2013-15-2934-00924) and all efforts were made to minimize suffering, distress and lasting harm.
Power calculations: Calculating effect size and power for this study was challenging due to the paucity of currently available data. For example, we could have used Kim et al.24 because of their use of the MCAo model and their investigation of depression-like behaviours, or Santiago et al.25 because of their use of a non-stroke model with motor deficits, but neither of these papers effectively report n numbers. A priori power analysis using sucrose preference testing data from our own publications on models of stress and depression, where the effect is substantial26, results in a Cohen’s d of 2.5. With a suggested power of 0.95 this gives a minimum samples size of 5.
Surgery: All surgeries were performed under isoflurane anaesthesia (2% in O2). The distal part of the left middle cerebral artery (MCA) was permanently occluded as previously described27,28. Briefly, an incision was made between the lateral part of the orbit and the external auditory meatus. A burr hole was made in the skull above the distal part of the MCA. The artery was occluded using bipolar forceps coupled to an electrosurgical unit (ICC 50, Erbe) causing local vessel coagulation, and ensuring a restricted cortical infarct. Animals received a local infusion of lidocaine at the injury site as well as subcutaneous saline/Temgesic and were allowed to recover in a heated environment for 30 minutes prior to being returned to normal housing conditions. No adverse events occurred during surgery.
Tissue collection and immunohistochemistry: Animals were surgically anaesthetized using pentobarbital (200 mg/ml) containing lidocaine (20 mg/ml; Glostrup Apotek, Denmark) and transcardially perfused with ice-cold 0.9% saline, followed by 4% paraformaldehyde. Brain tissue was cryoprotected in 30% sucrose and cut at 10µm in a coronal orientation. Individual series were stained with cresyl violet and infarct volumes were analysed using Aperio ImageScope (Aperio CS2 Scanner, Leica Biosystems, UK).
Behavioural tests: All behavioural tests were performed during the light phase following the NC3Rs guidelines and we have used the ARRIVE checklist when writing our report29. Behavioural analysis was videotaped and analysed blind to surgery.
Open field: This test was performed as previously described30 in a 45cm3 box with observations taking place over a 10 minute period. Movement was tracked using automated software (SMART v3.0, Panlab, Spain) connected to a camera positioned above the box. The area is divided into peripheral and central units, and locomotion and rearing can be recorded in these units. In the open field, low levels of engagement and interaction with the environment (staying close to the wall, moving around little) are traditionally thought to be traits describing emotionality31. Locomotion, rearing and time spent in certain predefined areas of the open field can be measured automatically, rearing was measured manually.
Marble burying: This test was performed as previously described32 in an open Perspex mouse home-cage (44 × 28 × 12cm) with 5cm of sawdust bedding. Twenty marbles were placed at 2cm intervals in 5 lines of 4. Animals were allowed to explore the cage for 30 minutes and the latency to start digging behaviours and the total number of marbles buried to at least 2/3 of their surface was counted. Latency was analysed manually post-hoc from video footage.
Tail suspension: Animals were individually suspended for a period of 6 minutes. Movement was analysed manually post-hoc from video footage. Immobility was defined as a period of no less than 3 seconds where no active movement was observed, escape activity was measured using a stop-watch and subtracted from the total time measured (6 minutes). Latency to the first period of consistent immobility (≥3 seconds) was recorded on a second stop-watch.
Sucrose preference: This test was performed as previously described33. Animals were acclimatized to sucrose with a 1% solution for 24 hours 7 days prior to surgery. No food or water deprivation was implemented. Baseline testing occurred the day prior to surgery. Mice were given 12 hours of free choice between either 1% sucrose or normal drinking water during the dark (active) phase of their cycle. This time frame excluded the possibility of using this test at the 6 hour survival time. The position of the solutions in the cage was switched at 6 hours to prevent side-bias. Percentage preference for sucrose was calculated at the end of the test using the following formula: Sucrose Preference = VolumeSucrose solution/(VolumeSucrose solution + VolumeWater) × 100.
Statistical analysis: All data were analyzed using Graphpad Prism software (version 7; GraphPad Prism Inc., La Jolla, CA). Analysis of data was performed using two-way analysis of variance (ANOVA) or one-way ANOVA, as appropriate. Data were considered significant at p<0.05. Tukey’s multiple comparisons post-hoc testing was applied as appropriate. All data sets are presented as mean ± standard error of the mean (SEM); n are included in figure legends.
In order to carry out basic characterization of the distal pMCAO model over a short time, we took brains at 6 hours, 24 hours, 2 days, 5 days and 7 days post-surgery. At no time point did sham animals show any significant CNS histopathology. Distal pMCAO animals showed a variation in infarct volumes over the time course, peaking at 24 hours (Figure 1A) with the infarct being restricted to the cortex at all time points. However, overall the ANOVA showed no statistical significance. Similarly weight loss in pMCAO animals was higher overall than sham animals (Figure 1B; two-way ANOVA; p<0.05) but no single time-point was significantly different from its sham counterpart.
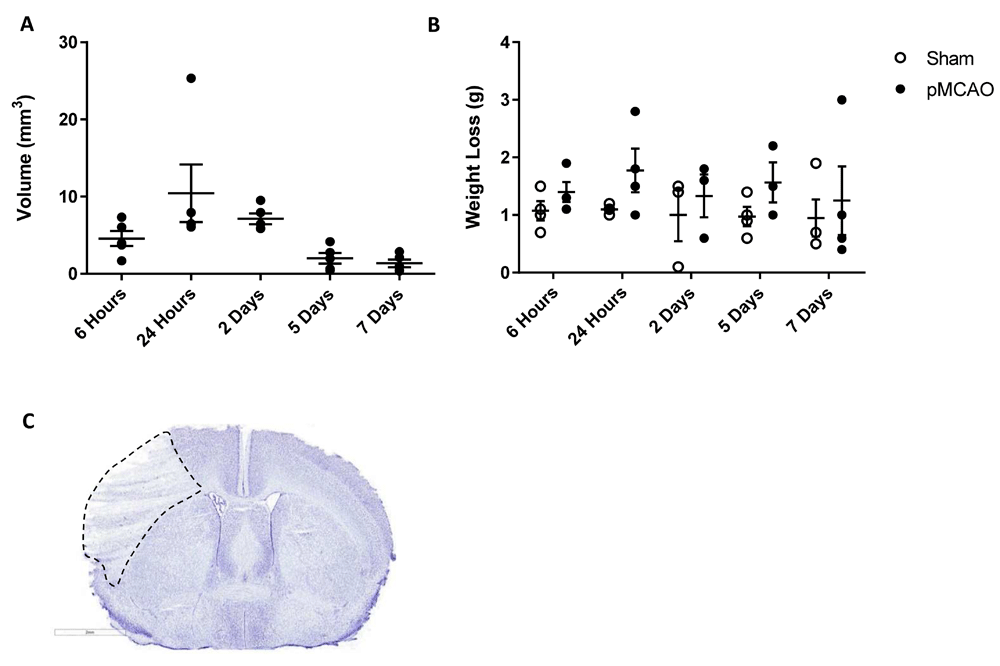
Animals underwent permanent occlusion of the distal middle cerebral artery and were allowed to survive for 6 hours, 24 hours, 2 days, 5 days and 7 days. Sham animals received surgery only with no occlusion of the artery. (A) Infarct volumes (mm3) in animals after surgery, sham animals showed no lesions. (B) Weight loss after pMCAO and sham surgeries. Data are presented as mean ±SEM, n=5.
Analysis of behaviour in the open field test (OFT) results in a number of useful metrics. Distance and speed demonstrate the capability for exploration, rearing behaviours show the motivation for exploration, and measuring the amount of time spent in the central zone vs the perimeter, shows the degree of anxiety the animal might be experiencing. Original studies of open field behaviour suggested that ‘emotional’ animals showed little interaction or engagement with their environments, indicative of a depression-like phenotype34. In our hands distance, speed and rearing were all generally below the levels shown by totally naïve animals (Figure 2A–C; represented by dotted line). Speed, distance and rearing behaviours were all reduced when pMCAO was taken as the main effect (Figure 2A–C; two-way ANOVA; p<0.05, p<0.05, p<0.01, respectively). In the majority of tests, no difference was found at any single time-point between pMCAO animals and their sham counterparts in post-hoc analyses. The exception being at 24 hours where rearing behaviour was significantly decreased compared to sham (Figure 2C; Tukey’s post-hoc test; p<0.05). At no point did animals change the amount of time they spent in the central zone vs the peripheral zone (Figure 2D).
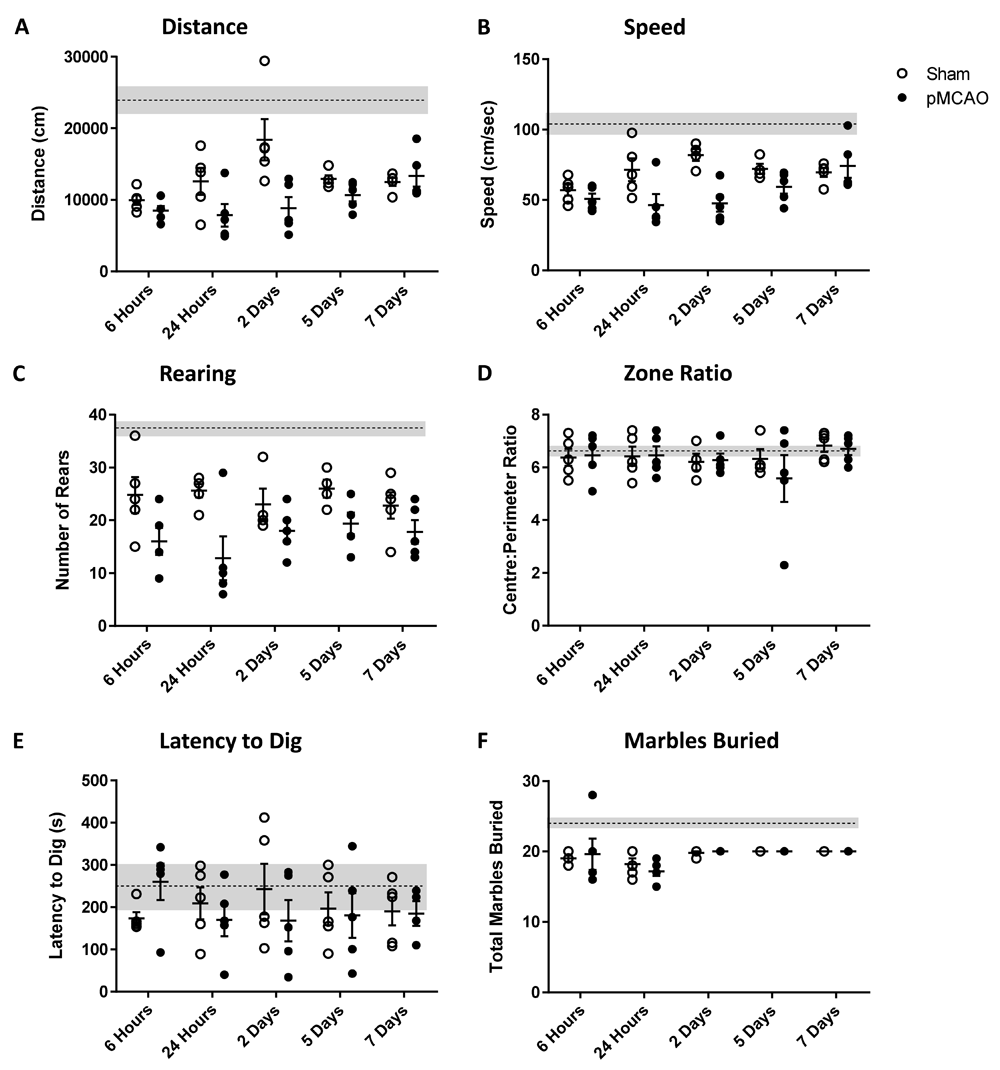
Animals underwent permanent occlusion of the distal middle cerebral artery and were allowed to survive for 6 hours, 24 hours, 2 days, 5 days and 7 days. Sham animals received surgery only with no occlusion of the artery. (A) Distance travelled in the open field (cm). (B) Speed travelled in the open field (cm/s). (C) Number of rears observed during the period of open field testing. (D) Ratio of time spent in the centre:perimeter areas of the open field. (E) Latency to begin digging in the marble burying test (sec). (F) Total number of marbles buried to at least 2/3 of their surface. Data are presented as mean ±SEM, n=5, dotted line represents naïve animals.
The digging behaviour seen in the marble burying test has been shown to be a motivational need in laboratory mice, rather than a direct measure of anxiety35,36. Here, all animals showed similar levels of motivation to dig the substrate material when compared to naïve animals, as measured by the latency to start digging (Figure 2E) and the total number of marbles covered in substrate (Figure 2F). At no point during the test were sham and pMCAO animals significantly different from each other.
The tail suspension test (TST) is a commonly used test to explore motivational escape-like behaviours in the study of depression37. Similarly, a lack of sucrose preference has been shown to be a good indicator of anhedonia, a depression-like sign in rodents21. In both the TST and sucrose preference tests there was no overall difference between post-surgery animals and naïve animals in any of the metrics analysed (Figure 3). In the TST, both latency to the first episode of immobility, and total duration of immobility were not affected by pMCAO, when these animals were compared to shams. Sucrose preference also did not decrease after surgery at any time. In order to determine whether preference was simply a facet of hypodipsia, total volume of liquid drunk was also measured. Whilst this was slightly decreased at 24 hours in all animals when compared to naïve, this did not reach significance, and at no time was the total volume of water missing from the bottles different in pMCAO and sham animals.

Animals underwent permanent occlusion of the distal middle cerebral artery and were allowed to survive for 6 hours, 24 hours, 2 days, 5 days and 7 days. Sham animals received surgery only with no occlusion of the artery. (A) Distance travelled in the open field (cm). (A) Latency to first period of immobility (≥3 seconds) in the tail suspension test. (B) total time spent immobile in the tail suspension test. (C) Percentage sucrose preference as measured over 12 hours. (D) Total fluid intake as measured over 12 hours. Data are presented as mean ±SEM, n=5, dotted line represents naïve animals.
The emotional disturbances associated with post-stroke depression can severely hinder recovery and, as such, are a crucial area for intervention. However, current anti-depressant therapies, even in uncomplicated major depressive disorder, are ineffective in up to 60% of patients38. Investigating new avenues for therapy in rodent models is a key way of addressing this. However, the rodent models used must be representative of the human condition. There is a long-held belief within the pre-clinical stroke community that the distal pMCAO model does not show any behavioural deficits which could constitute a depression-like phenotype but, to date, there are no citeable articles. This is likely due to a general publication bias in favour of positive data7,8. This study demonstrates that the distal MCAO model of stroke results in cortical lesions but no significant depression-like behaviours.
The distal pMCAO model produces a robust cortical lesion which varies slightly over time. This data is in line with others showing a large lesion at 24 hours22,28. This reduction in the manifest infarct is due to lesion resolution and the destruction of the core of dead tissue, largely by microglia and infiltrating macrophages39. The location of the lesion potentially contributes to the development, or lack thereof, of post-stroke depression, although results from studies thus far remain controversial40. In humans, there is some evidence for hemispheric bias but these results seem to be hampered by poor patient recruitment, inconsistent methodology and poor controls. Left-hemisphere lesions have been shown to be more commonly correlated with depressive symptoms than right hemisphere lesions41,42. However, a systematic review has shown that right-sided lesions may contribute in the sub-acute phase43. Whilst some degree of lateralization exists in the rodent brain44 the degree to which this contributes to emotionality, is still under scrutiny. Rather, the location of the lesion within the cortex may be the cause for the lack of an affective phenotype. Studies using the focal endothelin-1 (ET-1) model of stroke, where the ET-1 was introduced into the pre-frontal cortex, have shown depressive-like behaviours at the chronic time point (>1 week)45. The pre-frontal cortex is known to play a major role in depression in humans46,47 and whilst its existence in rodents is controversial48, the shift in location from the more functional areas of the cortex - such as the motor and sensory areas, to the PFC - may be the reason for the depression-like behaviours in the ET-1 model used by Vahid-Ansari and colleagues.
Emotionality in rodents has been traditionally challenging to study. Indeed, in models of depression controlling for slight variables in testing regime can significantly affect outcome21. This model of stroke has been repeatedly shown to have minor functional deficits in forepaw function20,22. In models of stroke, there has to be some consideration given to a lower degree of locomotor capacity. For example, the tail suspension test remains more appropriate than the forced swim test because of potential motor deficits shown by the MCAO animals. In this study, our data show that there is no significant change in anhedonic behaviours, either tail suspension or sucrose preference, even at 7 days post-stroke. In a bilateral model of global ischemia in the mouse, depression-like behaviours – as measured by sucrose preference and tail suspension - were not present at 7 days, but did develop at one month post-stroke49. Liu and colleagues suggest that neuronal loss within the hippocampus and hypothalamus may be responsible for this change in behaviour, and that these manifest at later time points in their model. It is possible that there could be some degree of persistent inflammation which continues after 7 days20, which may result in the development of depressive-like behaviours, but this was beyond the scope of this study.
In conclusion, this study demonstrates that whilst the distal permanent MCAO model produces a robust cortical lesion and is known to demonstrate markers of the ischemic cascade, relevant to interventional studies50, up to 7 days there are no significant affective components to this model. This study was powered using a different model of depression-like behaviours, using appropriate a priori power analysis (see methods). Post-hoc power analysis of the data suggests considerably more animals would be required to show an effective difference. For example, studying latency to immobility at 24 hours (where differences are likely to be most stark in the post-stroke brain) results in a suggested sample size of 161 animals to give a significant difference. Even in tests where the trend could potentially be seen, such as sucrose preference, the number of animals required to show a significant change was >40. Powering up pilot studies for negative results is an ongoing discussion amongst preclinical researchers and ethical review boards. Overall, this exploratory data provides citeable evidence that there is no overt depression-like phenotype in the acute phase of the distal MCAO model. The aim of providing this data is to inform those wishing to investigate depression-like behaviours post-stroke in rodents should consider using alternative models.
Dataset 1. Zip file containing all underlying behavioural and lesion volume data. https://doi.org/10.5256/f1000research.15769.d24983123
This work was funded by the Carlsberg Foundation [2012_01_0125 to YC and 2007_01_0176 to KLL].
The authors acknowledge the technical assistance provided by Louise Lykkemark and Dorte Lyholmer.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
References
1. Pothion S, Bizot JC, Trovero F, Belzung C: Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress.Behav Brain Res. 2004; 155 (1): 135-46 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Cressey D: UK funders demand strong statistics for animal studies. Nature. 2015; 520 (7547): 271-272 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 02 Jul 19 |
read | read |
|
Version 1 07 Sep 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
We appreciate the reviewers’ comments and additional suggestions. We also appreciate that this study is likely to be underpowered but given the data as it is, retrospective ... Continue reading Overall comment:
We appreciate the reviewers’ comments and additional suggestions. We also appreciate that this study is likely to be underpowered but given the data as it is, retrospective power calculations suggest an n of 45 for some of the tests covered, which we felt was inappropriate. The original plan for this research was to determine whether this was a good model for studying post-stroke depression. We originally used power calculations from sucrose preference testing from Couch et al., 2013 (Brain, Behaviour and Immunity) which was in a mouse model of depression. Calculating effect size and power for this study was challenging due to the paucity of currently available data. For example, we could have used Kim et al., (2016; BBR) because of their use of the MCAo model and their investigation of depression-like behaviours, or Santiago et al., (2014; BBR) because of their use of a non-stroke model with motor deficits, but neither of these papers effectively or consistently report n numbers. Our a priori analysis using our own model of depression-like behaviour indicated our sample size would be appropriate if we had a decent effect. After running the model and finding negative results, we presented this work as a poster at a conference and were repeatedly told our negative results were to be expected but the knowledge seemed to be largely word of mouth, not actively published. In order to prevent the experience we had for future researchers, we aimed to publish these data as an exploratory paper suggesting that, in the short term at least, depression-like behaviours are minimal in this particular model of stroke. It is likely that in the transient MCAo model, where the deficits are greater, or at a longer time-point, depression-like behaviours may exist.
For reviewers Albert and Vahid-Ansari:
1. One confound is whether multiple tests were done in the same day; this needs to be clarified: how many animals were used, what tests were done on each group, how many tests/day on each mouse?
We agree that multiple tests are likely to confound outcomes, but the usual outcome for this is to see a deficit where there isn’t one, rather than no deficit. The animals were all subjected to the same tests but in increasing order of severity; sucrose preference overnight followed by open field, followed by tail suspension. The sucrose preference was also post-surgery testing so minimal neophobia would have occurred through the introduction of the second bottle.
2. Fig. 1A: It would be important to show the size and location of the stroke at 24 hr. These strokes are notoriously variable, hence mapping the stroke is important. More details and quantification of the location (Bregma) of the incision and stroke need to be provided.
The image shown is the largest area of lesion at 24 hours and is representative of the model. This is the permanent middle cerebral artery occlusion model, employed using electrocoagulation at the distal site of the middle cerebral artery therefore the incision is made between the nose and ear on the side of the skull. ‘The variability of this stroke model is exceptionally low’ according to Llovera et al, in their 2014 (JOVE) methods paper on the use of this model. The representative image and lesion volume calculations are also standard practice for use in this model, where the spread of the lesion will be similar across all animals with minimal variability.
3. Since the stroke is so large and variable (esp. at 24 h), additional studies to assess fine motor impairments are critical (e.g., ladder test, cylinder test, etc. Motor impairments can affect results in locomotor, anxiety and depression tests.
We agree with the reviewer that motor deficits are likely to significantly affect psychological testing in rodent models which is why we did not aim to use tests which involved directed movement critical for survival or requiring more than simply generic movement, such as the hole-board memory test, or the forced swim test. It should be noted that the animals recovered from surgery extremely quickly and were found to be climbing upside down in the cage within an hour, irrespective of whether they were sham or stroke. The motor impairments in this model are well documented. In Lambertsen et al (2009; J.Neurosci) the authors use this model and demonstrate no alterations in foot pattern at 3 and 5 days post-surgery. In Madsen et al (2016; JCBFM) the authors demonstrate consistent but minor forepaw asymmetry at 3 and 5 days post-stroke. In Santiago et al (2014; BBR) the authors demonstrate in a model of Parkinson’s that when there is no obvious motor deficit in the open field, the depression-like behaviours studied are to be taken at face value. Here there is no obvious deficit in the open field and Lambertsen demonstrate only forepaw asymmetry. As forepaw dexterity is not required for any of the tests we have used for depression and anxiety, and the motor deficits have been extensively covered by others, we did not feel it necessary to extensive motor testing. However, we have added text to the discussion to cover these comments by the reviewer.
4. At none of the time points was any of the outcomes significant, only an overall significance. More data points are needed to test when there is impairment or recovery. Rearing is not specific measure for anxiety: validated tests need to be done (e.g., elevated plus maze, centre time in open field, etc.).
We appreciate the reviewers comments and additional suggestions. The use of rearing as an exploratory behaviour is based on our previous work. Valid anxiety tests were unfortunately unavailable to us at the time of this project but centre time in the open field has been included in figure 2D Centre:Perimeter ratio. The open field test is carried out over 10 minutes, this covers a short period of anxiety early in the test and then covers exploratory behaviour later. Neither early in the test, nor later, did we see any significant preference for either the centre or the perimeter of the open field.
5. Marble burying was also maximal 20 marbles for all groups, thus may be to insensitive to detect motor impairments.
As mentioned above, there were no significant motor impairments in the open field, and marble burying does not require the fine motor deficits which may occur as the result of this model. Indeed, the majority of burying behaviour, and burrowing behaviour, requires a combination of both forepaws and hindpaws. The marble burying test was carried out as commonly performed in the literature (Deacon et al., 2006; Nature Protocols).
6. In the sucrose preference test as performed, almost 100% of the mice show 100% preference for sucrose. This raises concern about the sensitivity of the test to detect anhedonia in C57BL6 mice: this strain of mice is unresponsive in this assay even after 9 weeks of chronic stress (Pothion, 2004). Hence the data are inconclusive.
In previous studies we have found no problems inducing deficits in sucrose preference after chronic stress in C57Bl6 mice (Couch et al, 2016, J.Neuroinflam.; Strekalova et al, 2015, BBR; Couch et al, 2013, BBI). We have developed the sucrose preference test and have found that pre-exposure to a very low concentration of sucrose (0.5%) over several days before the introduction of the baseline testing, gives greater preference for the sucrose solution. In addition, we have found that well-handled mice also give a greater response to sucrose. Working within these parameters we almost always achieve around 100% preference for sucrose. Indeed, we have used this test in more severe rodent models of motor deficit and found that even severely impaired animals are keen to drink sweetened liquids when trained and handled well. Overall we feel this is a valid test carried out well and the data are reliable. The animals did not show significant surgical impairment and so total fluid intake was not affected.
7. The data for TS are not convincing; the error seems too high since N value is too low. The dashed line needs to show N, mean ± SE of naïve mice: why are sham having more immobility than naïve?
We agree the error is high because of the low n, we have also included text within the manuscript outlining the spread of naïve data, which has also been added as an extra sheet in the excel raw data file. At 6 and 24 hours some deficit may be expected in all animals as this is still acutely post-surgery, irrespective of group. Sham animals have had all the associated surgery, merely without the occlusion. Despite the analgesia there may be some degree of discomfort which prevents them from being as active as naïve animals.
We appreciate the reviewers’ comments and additional suggestions. We also appreciate that this study is likely to be underpowered but given the data as it is, retrospective power calculations suggest an n of 45 for some of the tests covered, which we felt was inappropriate. The original plan for this research was to determine whether this was a good model for studying post-stroke depression. We originally used power calculations from sucrose preference testing from Couch et al., 2013 (Brain, Behaviour and Immunity) which was in a mouse model of depression. Calculating effect size and power for this study was challenging due to the paucity of currently available data. For example, we could have used Kim et al., (2016; BBR) because of their use of the MCAo model and their investigation of depression-like behaviours, or Santiago et al., (2014; BBR) because of their use of a non-stroke model with motor deficits, but neither of these papers effectively or consistently report n numbers. Our a priori analysis using our own model of depression-like behaviour indicated our sample size would be appropriate if we had a decent effect. After running the model and finding negative results, we presented this work as a poster at a conference and were repeatedly told our negative results were to be expected but the knowledge seemed to be largely word of mouth, not actively published. In order to prevent the experience we had for future researchers, we aimed to publish these data as an exploratory paper suggesting that, in the short term at least, depression-like behaviours are minimal in this particular model of stroke. It is likely that in the transient MCAo model, where the deficits are greater, or at a longer time-point, depression-like behaviours may exist.
For reviewers Albert and Vahid-Ansari:
1. One confound is whether multiple tests were done in the same day; this needs to be clarified: how many animals were used, what tests were done on each group, how many tests/day on each mouse?
We agree that multiple tests are likely to confound outcomes, but the usual outcome for this is to see a deficit where there isn’t one, rather than no deficit. The animals were all subjected to the same tests but in increasing order of severity; sucrose preference overnight followed by open field, followed by tail suspension. The sucrose preference was also post-surgery testing so minimal neophobia would have occurred through the introduction of the second bottle.
2. Fig. 1A: It would be important to show the size and location of the stroke at 24 hr. These strokes are notoriously variable, hence mapping the stroke is important. More details and quantification of the location (Bregma) of the incision and stroke need to be provided.
The image shown is the largest area of lesion at 24 hours and is representative of the model. This is the permanent middle cerebral artery occlusion model, employed using electrocoagulation at the distal site of the middle cerebral artery therefore the incision is made between the nose and ear on the side of the skull. ‘The variability of this stroke model is exceptionally low’ according to Llovera et al, in their 2014 (JOVE) methods paper on the use of this model. The representative image and lesion volume calculations are also standard practice for use in this model, where the spread of the lesion will be similar across all animals with minimal variability.
3. Since the stroke is so large and variable (esp. at 24 h), additional studies to assess fine motor impairments are critical (e.g., ladder test, cylinder test, etc. Motor impairments can affect results in locomotor, anxiety and depression tests.
We agree with the reviewer that motor deficits are likely to significantly affect psychological testing in rodent models which is why we did not aim to use tests which involved directed movement critical for survival or requiring more than simply generic movement, such as the hole-board memory test, or the forced swim test. It should be noted that the animals recovered from surgery extremely quickly and were found to be climbing upside down in the cage within an hour, irrespective of whether they were sham or stroke. The motor impairments in this model are well documented. In Lambertsen et al (2009; J.Neurosci) the authors use this model and demonstrate no alterations in foot pattern at 3 and 5 days post-surgery. In Madsen et al (2016; JCBFM) the authors demonstrate consistent but minor forepaw asymmetry at 3 and 5 days post-stroke. In Santiago et al (2014; BBR) the authors demonstrate in a model of Parkinson’s that when there is no obvious motor deficit in the open field, the depression-like behaviours studied are to be taken at face value. Here there is no obvious deficit in the open field and Lambertsen demonstrate only forepaw asymmetry. As forepaw dexterity is not required for any of the tests we have used for depression and anxiety, and the motor deficits have been extensively covered by others, we did not feel it necessary to extensive motor testing. However, we have added text to the discussion to cover these comments by the reviewer.
4. At none of the time points was any of the outcomes significant, only an overall significance. More data points are needed to test when there is impairment or recovery. Rearing is not specific measure for anxiety: validated tests need to be done (e.g., elevated plus maze, centre time in open field, etc.).
We appreciate the reviewers comments and additional suggestions. The use of rearing as an exploratory behaviour is based on our previous work. Valid anxiety tests were unfortunately unavailable to us at the time of this project but centre time in the open field has been included in figure 2D Centre:Perimeter ratio. The open field test is carried out over 10 minutes, this covers a short period of anxiety early in the test and then covers exploratory behaviour later. Neither early in the test, nor later, did we see any significant preference for either the centre or the perimeter of the open field.
5. Marble burying was also maximal 20 marbles for all groups, thus may be to insensitive to detect motor impairments.
As mentioned above, there were no significant motor impairments in the open field, and marble burying does not require the fine motor deficits which may occur as the result of this model. Indeed, the majority of burying behaviour, and burrowing behaviour, requires a combination of both forepaws and hindpaws. The marble burying test was carried out as commonly performed in the literature (Deacon et al., 2006; Nature Protocols).
6. In the sucrose preference test as performed, almost 100% of the mice show 100% preference for sucrose. This raises concern about the sensitivity of the test to detect anhedonia in C57BL6 mice: this strain of mice is unresponsive in this assay even after 9 weeks of chronic stress (Pothion, 2004). Hence the data are inconclusive.
In previous studies we have found no problems inducing deficits in sucrose preference after chronic stress in C57Bl6 mice (Couch et al, 2016, J.Neuroinflam.; Strekalova et al, 2015, BBR; Couch et al, 2013, BBI). We have developed the sucrose preference test and have found that pre-exposure to a very low concentration of sucrose (0.5%) over several days before the introduction of the baseline testing, gives greater preference for the sucrose solution. In addition, we have found that well-handled mice also give a greater response to sucrose. Working within these parameters we almost always achieve around 100% preference for sucrose. Indeed, we have used this test in more severe rodent models of motor deficit and found that even severely impaired animals are keen to drink sweetened liquids when trained and handled well. Overall we feel this is a valid test carried out well and the data are reliable. The animals did not show significant surgical impairment and so total fluid intake was not affected.
7. The data for TS are not convincing; the error seems too high since N value is too low. The dashed line needs to show N, mean ± SE of naïve mice: why are sham having more immobility than naïve?
We agree the error is high because of the low n, we have also included text within the manuscript outlining the spread of naïve data, which has also been added as an extra sheet in the excel raw data file. At 6 and 24 hours some deficit may be expected in all animals as this is still acutely post-surgery, irrespective of group. Sham animals have had all the associated surgery, merely without the occlusion. Despite the analgesia there may be some degree of discomfort which prevents them from being as active as naïve animals.