Keywords
Contact dermatitis - Cardiac Pacemaker - Patch Tests – Polytetrafluoroethylene.
Contact dermatitis - Cardiac Pacemaker - Patch Tests – Polytetrafluoroethylene.
Pacemaker system hypersensitivity is a rare complication of cardiac pacing and diagnosis is usually difficult, and is often delayed1. In fact, bacterial infection is initially suspected routinely rather than allergy to the pacemaker components1. Management of this condition is difficult and not well established.
Here, we report a patient who developed repeated sterile skin necrosis leading to generator externalization. Prick testing was of a doubtful interpretation. The patient recovered after implantation of a poly-tetra-fluoro-ethylene (PTFE)-coated pacemaker generator in a retropectoral position.
A 75-year-old man with symptomatic complete atrioventricular block received a dual chamber pacemaker (MEDTRONIC SIGMA DR) in November 2000. The patient required two generator re-implantations in March 2005 and August 2007 due to sterile skin necrosis with externalization of the pacemaker generator. A St Jude Medical IDENTITY DR pacemaker was used in the two replacements.
In May 2009, the patient presented with a third externalisation of the pacemaker generator, with localized swelling and redness in the implanted area. The patient had no fever, and inflammatory parameters during blood testing were negative; except the patient had a high eosinophil fraction (0.59×103/L; normal range: 0.0–0.5 ×103/L). Blood cultures, bacterial swabs and cultures of the material taken gave negative results. Swabs from pacemaker pocket showed that C-reactive protein and procalcitonin were negative. We performed a skin prick test using a standard battery (not including titanium). Little positivity was found to nickel and chrome batteries, but skin application of another pacemaker generator (St Jude Medical IDENTITY DR) produced a suggestive skin response.
The patient underwent removal of the old pacemaker system and re-implantation of a new pacemaker system. The generator (Saint Jude Medical Victory XLDR) was entirely coated with PTFE sheet and implanted in the left chest wall in a retro pectoral position (Figure 1). The procedure was uneventful and the patient was discharged in good condition.
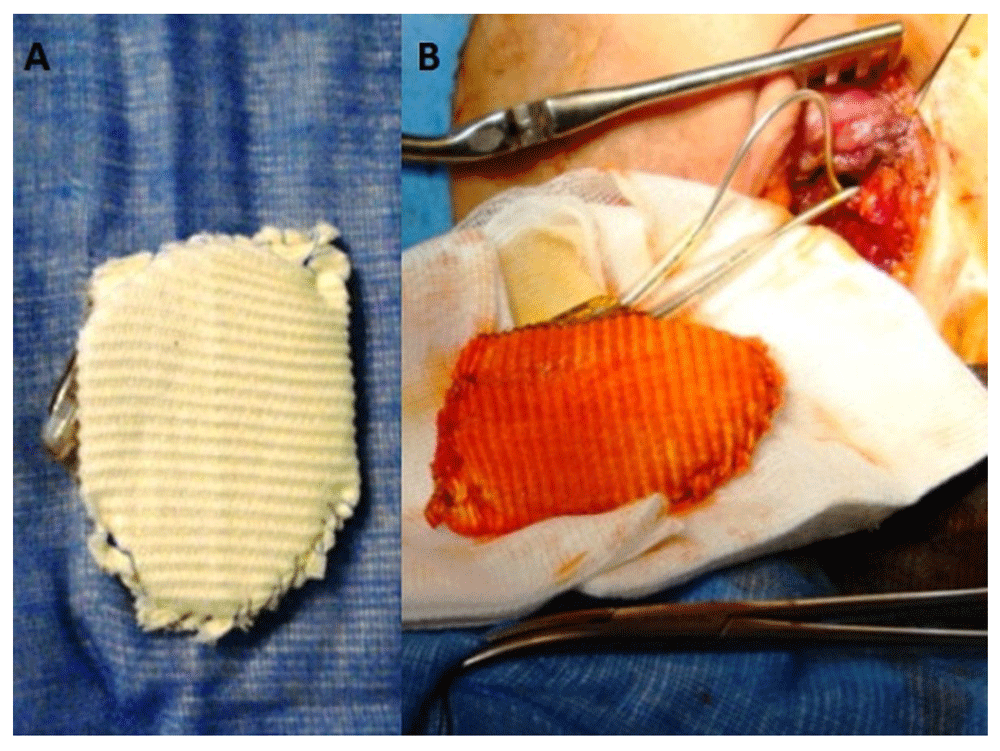
(A) Entirely coated pacemaker generator with PTFE sheet; (B) coated generator connected to two leads implanted in retropectoral position.
After 23 months of regular follow-up, the generator moved from a retropectoral to a subcutaneous position on chest CT scan. No recurrence of externalization or cutaneous signs was observed up to nine years after implantation
Pacemaker component allergy is a rare but well established complication of cardiac pacing. It was first reported by Raque and Goldschmidt2 in 1970 in a patient presenting with eczematous dermatitis developed overlying the pacemaker site within 3 weeks of implantation. Since then various reports have been published3,4. Several clinical presentations are observed varying from local pain to systemic manifestations. Pacemaker-mediated dermatitis is thought to be a delayed hypersensitivity type 3 or 4 mediated reaction5. The time taken for sensitivity to develop varies from months to years5. In our case, the allergic process occurred several years after implantation. The allergen can be located in the “CAN” or other components of the pacing system. Titanium, nickel and epoxy resin are the most common allergens6.
Diagnosis of pace maker allergy is difficult and infection must be ruled out before any hypersensitivity investigation. Skin patch tests are helpful but not always contributive since their sensitivity is not very high. In our patient, skin tests showed a doubtful reaction to nickel and chrome. Titanium is the main component of pacemaker generator, is not included in the standard battery and was very likely to be an allergen because skin reaction was only observed above the generator. Otherwise, Déry and colleagues6 suggested that titanium test is unreliable because this test is performed using titanium tetrachloride, which must be highly diluted with water and quickly hydrolyzed to insoluble titanium dioxide. A positive reaction to nickel can be found in up to 20% of the population7; therefore nickel tests should be interpreted with caution. Furthermore, skin tests reading can be extended to 72 hours to have more positive results8. A positive reaction to intracutaneous testing on the affected patient’s serum incubated with small pieces of titanium for 1 month in a patient who had negative results on patch testing to titanium was reported by Yamauchi et al.9. In our patient, clinical presentation is very suggestive of hypersensitivity and titanium is very likely to be an allergen because skin reaction was only observed above the generator (composed mainly of titanium).
Treatments of contact dermatitis have been described in various case reports. There is a role for topical corticosteroid that can reduce skin symptoms, but recurrence is common10. The only complete treatment is the removal of the allergen, and the use of a hypoallergenic material. One option, as described by Syburra et al. and Andrews and Scheinman1,11, is the use of gold-coated generator. PTFE sheet coating technique was first reported in Japan8,12–14. This technique seems to be an effective method despite theoretical risk of PTFE hypersensitivity. In our patient, lack of recurrence confirms pacemaker component allergy and effectiveness of PTFE coating to prevent it.
Although pacemaker allergy is a rare condition, its recognition is important considering the widespread use of pacing and defibrillation systems. Allergic reaction can occur early or be delayed. It is recommended to rule out this diagnosis in the case of pacemaker pocket inflammation without signs of an infection. A negative skin test should not exclude diagnosis. We report in the present case that removal of the system components and the use of PTFE-coated materials are effective to prevent recurrence with a long follow-up.
Written informed consent for publication of the clinical details and images was obtained from the patient.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: EHra EP specialist (electrophysiologist)
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Sep 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)