Keywords
alarmin cytokine, microRNA, schistosomiasis
alarmin cytokine, microRNA, schistosomiasis
Schistosomiasis is one of the most prevalent, but neglected, tropical infectious diseases, afflicting more than 240 million people in 78 countries, including children and young adults1. It is caused by trematode parasites of the genus schistosoma, of which three are known to cause severe disease in humans, namely Schistosoma mansoni (S. mansoni), Schistosoma japonicum, and Schistosoma haematobium. S. mansoni and S. japonicum result in intestinal schistosomiasis, whereas S. haematobium is responsible for urinary schistosomiasis. Current strategies to control this disease rely heavily on the administration of praziquantel2. However, its widespread use may result in the development of drug resistance3. Vaccination is a good strategy to control or eradicate this disease; however, an effective vaccine is still lacking because of our limited knowledge of the immunological mechanisms associated with the elimination of the pathogen4. In addition, hepatic fibrosis is the primary cause of morbidity and mortality from schistosomiasis, but the disease mechanism remains elusive, and effective intervention is lacking1. A better understanding of the mechanism of the host–schistosome interaction is the key to solving these problems. Accumulating evidence shows that alarmin cytokines and microRNAs (miRNAs) are crucial regulators in the host–schistosome interaction. In this review, we summarize the research advances in these fields.
Schistosomiasis is an immune pathological disease. The type 2 immune response, characterized by the T helper 2 (Th2) cell-associated cytokines, such as interleukin 4 (IL-4) and IL-13, is the central regulator of disease progression in schistosomiasis5. However, the signals that drive the type 2 immune response after infection remain largely unknown6. Numerous studies have highlighted that tissue damage, which induces the release of alarmin cytokines, including IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), is a potent mechanism driving type 2 immunity7, particularly in the context of helminth infection8. TSLP regulates dendritic cells, basophils, mast cells, monocytes, natural killer T cells, and type 2 innate lymphoid cells (ILC2s)9, whereas IL-25 and IL-33 exhibit similar Th2-promoting activity largely by stimulating ILC2s, basophils, mast cells, and eosinophils10,11. The role of alarmin cytokines in schistosomiasis has been intensively studied; however, published results have been inconsistent12–15.
Serum IL-33 was observed to be markedly increased in patients infected with S. japonicum16, whereas serum IL-33 and intracellular IL-13 in eosinophils were elevated in patients infected with S. haematobium after chemotherapy17. In addition, serum soluble ST2, a decoy receptor of IL-33, was significantly elevated in patients with end-stage S. japonica infection, whereas membrane ST2 expression was obviously increased in the fibrotic liver tissues18. In addition, ST2 genetic variants were strongly associated with serum soluble ST2 levels18. In a mouse model of schistosomiasis, ST2 deficiency or blockade of IL-33 using soluble ST2 treatment or neutralizing antibodies showed significantly less Th2-mediated pathology, including marked decreases in granuloma and fibrosis formation, or Th2 cytokine production12,13,19. Decreased pulmonary collagen deposition and granuloma size were also observed in mice deficient in IL-25 or its receptor following S. mansoni egg challenge14. In addition, IL-33 regulated hepatic type 2 pathology after schistosome infection by promoting the production of type 2 macrophages (M2)20. Our group found that hepatic stellate cells (HSCs) were the primary source of IL-33 and that ILC2s were the primary source of IL-13 in the infected mouse livers21. However, two other groups proved that IL-33– or IL-25–deficient mice exposed to schistosome eggs or cercariae showed no significant differences in granuloma size and collagen deposition15,22. Instead, marked reductions in granuloma size and fibrosis extent were observed when IL-25, IL-33, and TSLP were simultaneously disrupted15. In addition, primary, but not secondary, granulomatous inflammation in the lungs challenged with eggs was reduced in TSLP receptor–deficient mice, and hepatic fibrosis instead of granuloma was reduced in these mice23.
These studies seemed to imply that the roles of TSLP, IL-25, and IL-33 in the initiation of type 2 immunity induced by schistosome infection were redundant, and single alarmin cytokine had little impact on the course of schistosome infection. Given the potential therapeutic value of these alarmin cytokines in controlling Th2 pathology, further research is needed to determine their roles in schistosomiasis. It appears that the inconsistency in the published results might be caused by the differences in intervention time, especially for IL-33. When IL-33 was depleted in the embryo stage, the role of IL-33 might be compensated for by other factors; however, when IL-33 was neutralized during disease progression, the role of IL-33 in disease progression became more obvious.
Over the last decade, miRNAs have emerged as important regulators of human diseases24. MiRNAs are endogenous, small non-coding RNAs that negatively regulate post-transcriptional gene expression through binding with partial complementarity to their target mRNA sequences25. In schistosomiasis, miRNAs have been increasingly studied for their potential roles in host–parasite interactions.
MiRNA deregulation is a hallmark of a variety of human diseases, including infectious diseases. Identification of host-deregulated miRNA during infection may uncover novel disease mechanisms or potential therapeutic targets. Alterations in host miRNAs following schistosome infection have been studied extensively. Hepatic fibrosis is the primary cause of morbidity and mortality from human schistosomiasis, and HSCs are the main effector cells for hepatic fibrosis26. Via a miRNA microarray, a series of host miRNAs have been identified that were deregulated during the progression of hepatic fibrosis in a mouse model of S. japonicum infection. Importantly, adeno-associated virus 8 (AAV8)-mediated inhibition of those host miRNAs, such as miR-21 and miR-351, or elevation of miR-203 significantly protected hosts from lethal schistosomiasis via attenuation of hepatic fibrosis21,27,28. HSCs were target cells of these three crucial host miRNAs. In HSCs, miR-21 was induced by transforming growth factor-beta 1 (TGF-β1) and IL-13, the primary cytokines responsible for fibrosis induced by schistosome infection29. In addition, elevated miR-21 prompted TGF-β1/SMAD and IL-13/SMAD signaling to induce fibrogenic effects by relieving the inhibitory effect of SMAD7 in the SMAD pathway. MiR-351 levels are elevated during the infection and the increased miR-351 promoted the activation of HSCs by targeting the vitamin D receptor, a newly identified negative regulator of the SMAD pathway30. Moreover, the schistosome infection downregulated miR-203 expression, which relieved the inhibition of IL-33, and sequentially elevated levels of IL-33 were released into the liver tissue, which stimulated the proliferation and IL-13 production of hepatic ILC2s (Figure 1A). In addition, Zhu et al. found that miR-454 promoted the activation of HSCs after infection by targeting SMAD431. Importantly, there is evidence that host miRNAs played an important role in regulating hepatic fibrosis in humans infected with S. japonicum32.
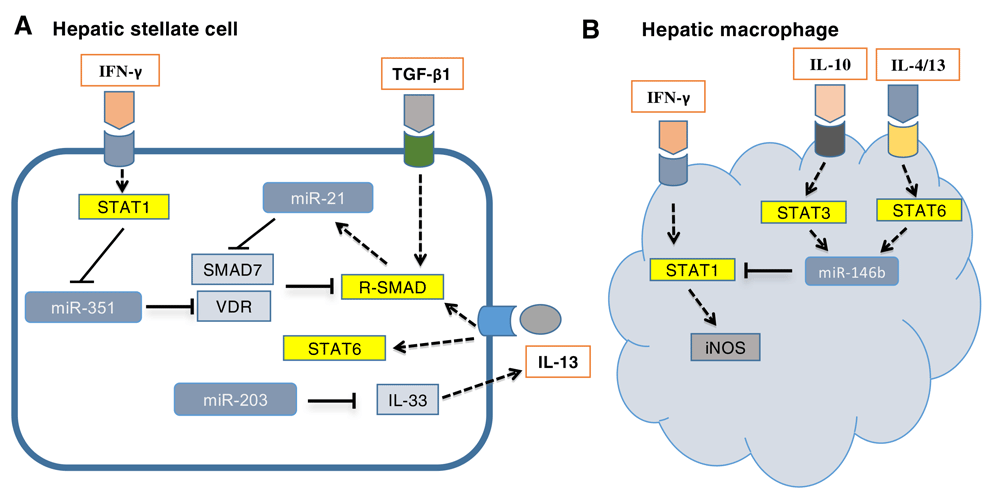
(A) Transforming growth factor-beta 1 (TGF-β1) and interleukin-13 (IL-13) are the primary cytokines responsible for fibrosis induced by schistosome infection by activation of SMAD pathway. In hepatic stellate cells, miR-21, induced by TGF-β1 and IL-13, prompted TGF-β1/SMAD and IL-13/SMAD signaling to induce fibrogenic effects by relieving the inhibitory effect of SMAD7 in the SMAD pathway. MiR-351 was negatively regulated by interferon-gamma (IFN-γ) via activation of the STAT1 pathway and promoted fibrogenesis by targeting vitamin D receptor (VDR), a newly identified negative regulator of the SMAD pathway. Moreover, infection-induced downregulation of miR-203 expression resulted in hepatic fibrosis by relieving the inhibition of IL-33, which elevated the expression of IL-13. (B) In hepatic macrophages, various T helper 2 (Th2) cytokines, such as IL-4, IL-13, and IL-10, promoted the transcription of miR-146b by activating STAT3/6, and elevated miR-146b inhibited the IFN-γ–induced differentiation of macrophages into M1 cells by targeting STAT1. iNOS, inducible nitric oxide synthase.
Schistosomiasis is an immune pathological disease, and various types of immune cells, especially macrophages and T cells, play an important role in disease progression. We found that elevated miR-146b inhibited the interferon-gamma (IFN-γ)-induced differentiation of macrophages into M1 cells by targeting STAT1 (Figure 1B)33. Kelada et al. showed that miR-182 critically prevented IL-2 production in Th2-associated regulatory T (Treg) cells34. These studies highlighted that miRNAs are crucial regulators in the initiation and progression of schistosomiasis, and targeting the deregulated miRNA is a potential therapeutic intervention for this chronic disease.
The interplay among various cytokines, including both Th1 and Th2 cytokines, has a crucial role in the initiation and progression of schistosomiasis. IL-13 and TGF-β1 are the effector cytokines of hepatic fibrosis induced by schistosome, whereas IFN-γ has anti-fibrotic activity in this disease29. IL-4 is responsible for the formation of egg granuloma, and IL-10 is the main negative regulator of pathology6. Interestingly, these cytokines also play a crucial role in the regulation of host miRNA expression during schistosomiasis. IL-13 and TGF-β1 additively upregulated the expression of miR-21 in HSCs by activating SMAD proteins, which promotes the maturation of miR-2127. IFN-γ inhibited the expression of miR-351 in HSCs through a pathway dependent on the transcription factor STAT1 to induce the expression of interferon regulatory factor 2 (IRF2), which binds to the promoter of pre-miR-351 to inhibit its transcription28 (Figure 1A). Various Th2 cytokines, including IL-4, IL-10, and IL-13, could induce macrophages to express miR-146b by activating STAT3/6, which bind to the promoter of the pre-miR-146b gene and initiate its transcription33 (Figure 1B). In Treg cells, IL-4 regulated miR-182 by inducing cMaf, an IL-4–regulated transcription factor34. These studies uncovered the mechanisms by which cytokines regulate disease progression and further highlighted their crucial roles in this disease.
The availability of parasite genome sequences, combined with advances in RNA sequencing, has paved the way to identify novel miRNAs in schistosomes. The presence of miRNAs in S. japonicum was first reported by our group through cloning and sequencing a small (18- to 26-nucleotide) RNA cDNA library from adult worms35. Subsequently, detailed information of miRNA expression in this parasite was generated through the analysis of small RNA libraries from particular developmental stages36. As of 25 June 2018, a search for S. japonicum and S. mansoni indicated that 171 pre-miRNAs are currently annotated in miRBase (www.mirbase.org/search.shtml). The expression of many of these parasite miRNAs is stage-, gender-, or cell type-specific, implying their crucial roles in parasite development and sex maturation37. Targeting these miRNAs and their regulated genes or pathways should be a novel strategy for disease control, but it is difficult to implement this strategy because of limited genetic manipulation for the parasite38. However, miRNAs are a highly conserved group of small RNA molecules, expressed by most organisms, and have similar mechanisms of miRNA function. Recent studies showed that miRNAs could be released from pathogens, such as bacteria and parasites, living in the hosts39–41. These cell-free miRNAs are very stable in the host body fluids and can be absorbed by distant host recipient cells, which suggest that pathogen-derived miRNAs can regulate the function of host cells in a cross-species manner. This new manner of host–parasite interaction has been validated in an animal model infected with Heligmosomoides polygyrus42. The miRNAs contained in parasite exosomes suppressed type 2 innate immunity in mice. Accumulating evidence shows that all stages of the schistosome could secrete exosome-like vesicles that contain numerous parasite miRNAs and that these vesicles could transport their cargo miRNAs to host cells, where the parasite-derived miRNAs regulated host gene expression, thereby exerting their effects on the occurrence and progression of host disease during infection43–45.
The type 2 immune response is the central regulator of disease progression in schistosomiasis. The role of alarmin cytokines in schistosomiasis has been intensively studied; however, the results from different studies have been inconsistent. Further research is needed to address these inconsistences. Host miRNAs are crucial regulators of disease progression and targeting host-dysregulated miRNAs is a potential strategy to treat this chronic disease. Parasite infection induces the expression of certain cytokines that regulate the expression of host miRNAs through various signaling pathways, which contribute to modulating the occurrence and progression of host diseases. Parasite-derived miRNA-mediated cross-species regulation of host genes might promote various disease processes or strengthen host resistance to the diseases. These studies have already broadened our understanding of the mechanisms of host–schistosome interaction and may be translated into new therapeutic targets.
This work was supported by grants from the National Natural Science Foundation of China (81430051).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 28 Sep 18 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)