Keywords
ROS, photochemistry, methylene blue, degradation, UV
ROS, photochemistry, methylene blue, degradation, UV
Consistent access to clean water has come into focus this millennium due to high pollution; a reduced amount of drinkable water could be the next challenge for the future due to overpopulation1–3. The application of photocatalytic technology using semiconductors to solve the environmental problems, like the degradation of organic effluents have been received much attention4–8. Heterogeneous photocatalysis using semiconductors is an interesting method falling into advance oxidation processes (AOPs)9–11 that can produce highly reactive species containing oxygen (ROS). In fact, with this method is possible to produce oxidizing molecules like hydrogen peroxide and singlet oxygen (1O2) together with radicals like hydroxyl radical (OH.) and superoxide radical anion ( O2.- )12–13. These reactants can decompose organic pollutants in wastewater giving harmless compounds14.
Recently, N. Chen et al. reported that reactive oxygen species generation in hydrochar and photochemistry of Sulfadimidine degradation in water15. Y. Chen et al. reported the photo degradation of tetracycline in aqueous solution under simulated sunlight irradiation through the singlet oxygen16. Li et al. reported that the degradation of ibuprofen by UV–visible light irradiation included direct photolysis and self-sensitization via ROS17. Wang et al. reported that when a simpler molecule without visible-light absorption is degraded, the Fe-hydroxyl complexes still promote the generation of ROS and thus accelerate degradation, although the pathway of electron transfer, and the mechanism of photocatalysis was not completely understood18.
In literature are present many methods for photoassisted AOPs like photo-electrochemical cells composed by an anode made with boron-doped diamond and cathode in carbon nanotubes; with this system, a model azo dye was depleted19. Also exfoliated graphene, decorated with titanium dioxide and nanoparticles, is effective for photo-catalytic water treatment20,21.
In our current scenario, stable peroxyl radicals in carbon-supported silica (PCS) are prepared from cheap starting materials. The method used is the pyrolysis under vacuum of kraft lignin deposited onto silica. Vacuum pyrolysis produced defective carbon bearing carbon radicals. These radicals are quickly transformed into peroxyl radicals by reaction with oxygen molecules present in the atmosphere.
The materials and methods to produce PCS using high-vacuum pyrolysis are clearly explained and characterized previously22. In brief, kraft lignin was absorbed onto silica and pyrolyzed under vacuum at 600 °C. For the kinetic data analysis, linear quadratic fitting and other kinetic fitting (reaction order checking) were performed by using Origin v6.0.
100-ml of air-equilibrated 10-6 M solutions of MB (Sigma Aldrich, India) in water containing 100 mg (1 mg/ml) of neat SiO2 or PCS were poured in quartz cylindrical reactors (90 mm diameter x 25 mm height). Solutions were magnetically stirred in the dark for 10 min before irradiation and kept under stirring during the experiment. The light source consisted of two 15-W phosphor-coated lamps (center of emission, 366 nm). Aliquots (4 ml) were withdrawn at 5-min intervals (for a total of 10-12 samples) during the irradiation until the disappearance of the color. Solids were removed by syringe filtration with a 0.4-µm pore size, and the filtrates immediately examined by UV-visible absorption spectroscopy in 1-cm quartz cuvettes using a JASCO V-630 UV-visible spectrophotometer. The absorbance was normalized by dividing the absorbance at 668 nm of the sample (A) with the absorbance of the initial solution (A0).
To assess the respective photocatalytic activity of PCS and of neat SiO2, we carried out competitive experiments with MB (Figure 1). PCS did not react with MB, in fact, solutions left for 24 hours in the dark does not show a decrease of MB concentration. Nonetheless, under dark conditions the dye was absorbed by PCS to a nearly tenfold greater extent than with pristine SiO2 (dark region between −10 and 0 min, Figure 1b).
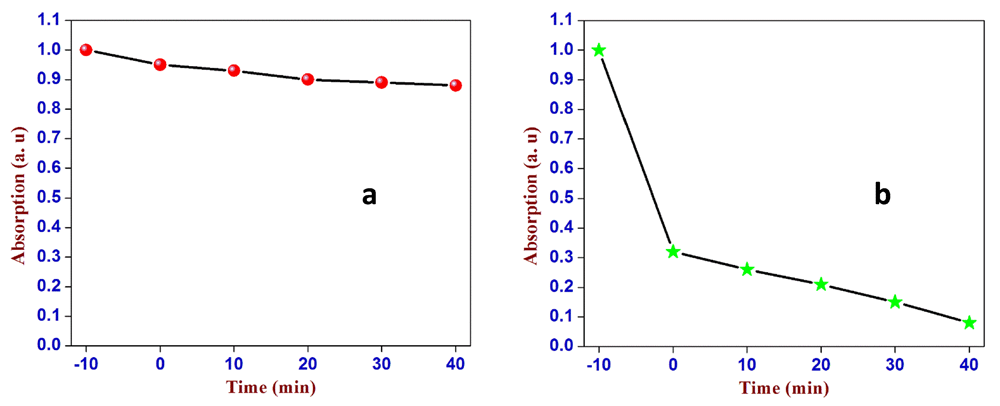
Normalized spectral intensity of the 668 nm band of methylene blue (MB) during (a) the UV-irradiation of the MB/SiO2 suspension at 366 nm at different time intervals, and (b) the same process for the MB/peroxyl radicals in carbon-supported silica (PCS) suspensions under otherwise identical conditions. The region between −10 and 0 min refers to the extent of adsorption of the MB dye under dark conditions. It shows the first-order kinetics of the photodegradation of the MB dye by MB/PCS. 3 repeats performed.
Normally photocatalysts produce radicals able to degrade organics but in the case of PCS the catalyst already possesses reactive radicals.
The net effect of PCS on the photodegradation of MB is a threefold increase in the kinetics of photodegradation (Table 1). Without the assistance of an active photocatalyst, the only reaction mechanism that is applicable is the generation of singlet oxygen by sensitization (Equation 2) via the excited state of the dye. The singlet oxygen can react with MB, giving rise to photobleaching (Equation 3).
| Dye | k (min−1) | Adsorption, % | ||
|---|---|---|---|---|
| SiO2 | PCS | SiO2 | PCS | |
| MB | 0.027 ± 0.005 | 0.092 ± 0.006 | 24 | 91 |
Dye + photon = Dye* (1)
Dye* + O2T =Dye + O2s (2)
O2S + Dye = oxidation products (3)
With PCS, MB is strongly absorbed onto the pyrolytic carbon present on the catalyst surface. Moreover, pyrolytic carbon possesses a high concentration of peroxyl radicals. The enhancement on the reaction kinetic could be due to a local increase of concentration of dye and active oxygen. Since the oxygen is reversibly absorbed on the carbon giving peroxyl radicals22, the surface of the catalyst is never depleted due to the presence of oxygen in solution.
In fact, in these conditions, we can have, together with Equation 1–Equation 3, a possible reaction of the excited state of the reactant with peroxyl radicals or adsorbed oxygen on PCS (Equation 4).
Dye* + PCS-OO = PCS + dye oxidation (4)
The peroxyl radicals are reversibly formed by capture of atmospheric oxygen due to the presence of highly active pyrolytic carbon on PCS:
PCS + O2 = PCS-OO (5)
Another possibility is the transfer of energy (or sensitization) of the excited state of the absorbed dye directly to the defective pyrolytic carbon, giving rise to formation of ROS. All these mechanism lead to an enhancement on the degradation of MB.
This study has shown that silica can be coated successfully with pyrolytic carbon obtained from inexpensive waste materials, such as kraft lignin and silica. The pyrolytic process performed at 600°C did not affect the crystalline state of silica when it was coated with carbon. The photocatalytic activity was measured against pristine SiO2 through an examination of the kinetics of degradation of MB by UV-vis spectroscopy. Under UV light irradiation, the degradation was threefold greater for the MB-PCS compared with MB-silica.
Dataset 1: Raw data for the article ‘Pyrolytic formation and photoactivity of reactive oxygen species in a SiO2/carbon nanocomposite from kraft lignin’ are presented, 10.5256/f1000research.16080.d21890723
We are grateful to the PANACEA - ERASMUS MUNDUS of the European Commission within the project Agreement Number 2012-2647/001-001 - EMA2 for an Action 2 scholarship in support of D.V.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We wish to thank Prof. Nick Serpone of the PhotoGreen Laboratory of the Department of Chemistry at the University of Pavia for useful discussions.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am currently working in photochemistry, photocatalysis, and elucidation of reaction mechanisms on the ground and excited state.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Sep 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)