Keywords
Benign, pediatric, radiation therapy, surgery, outcomes, treatment side effects
Benign, pediatric, radiation therapy, surgery, outcomes, treatment side effects
Craniopharyngioma is a benign tumor typically treated with both surgery and radiation, an approach that offers 5-year progression-free survival (PFS) rates exceeding 90%1. Historically, these high tumor control rates have come at the cost of long-term side effects, such as endocrinopathy, hypothalamic dysfunction, visual field deficits, cerebrovascular sequelae, secondary malignancies, and neurocognitive decline, which significantly impact quality of life among this mostly pediatric population.
Whereas most benign tumors can be treated surgically, craniopharyngiomas present a surgical challenge because of their central location and close proximity to sensitive structures, such as the optic apparatus, pituitary, hypothalamus, circle of Willis, brain stem, and temporal lobes. Schoenfeld et al. retrospectively reviewed 122 patients whose craniopharyngioma was treated between 1980 and 2009 with gross total resection (GTR) or subtotal resection (STR) and radiotherapy2. GTR was associated with a significantly higher incidence of diabetes insipidus (56.3% versus 13.3%, p <0.001) and panhypopituitarism (54.8% versus 26.7%, p = 0.014) and showed no improvement in PFS or overall survival2. In an analysis of 644 patients from the Surveillance, Epidemiology and End Results Program whose craniopharyngioma was treated between 2004 and 2008, Zacharia et al. examined factors such as younger age, smaller tumor size, and combined-modality therapy3. They found that STR and radiotherapy significantly improved survival. In addition, the 10-year local control rate was higher with STR plus radiation than with surgery alone (84% versus 52%; p = 0.006)3. STR alone has been associated with significantly inferior PFS compared with surgery and radiation2. Because aggressive surgery carries a higher risk of morbidity and the rates of progression with STR alone are unsatisfactory, the standard of care for most craniopharyngiomas involves conservative surgery with the goal of preserving vision and controlling hydrocephalus, followed by radiotherapy to optimize local control. Conservative surgical resection is of particular importance in cases with radiographic evidence of hypothalamic involvement, which is associated with decreased 10-year overall survival and an enduring impact on psychosocial quality of life4,5. Only a select subset of tumors, usually small and separate from the hypothalamus and optic pathway, may be cured with surgery alone.
The most recent advances in the treatment of craniopharyngioma have focused on minimizing treatment-related toxicity. These advances include endoscopic surgery and precision radiotherapy. Radiation therapy technology has improved dose conformality and provided decreased doses to adjacent critical structures with the goal of reducing long-term sequelae in this highly curable pediatric population.
Prior to the advent of endoscopy, only intrasellar, infradiaphragmatic lesions could be resected through an endonasal approach. With the advent of endoscopic endonasal surgery (EES), suprasellar and select intraventricular tumors, which were accessible only using craniotomy, can now be resected using EES, often with improved clinical outcomes compared with transcranial resection. Karavitaki et al. reviewed 64 craniopharyngioma patients who underwent EES6. The GTR and near total resection rates were 37.5% and 34.4%, respectively, similar to historical rates with transcranial resection6. There was no difference in extent of resection between intrasellar and suprasellar tumors. The rates of visual deterioration (0%) and new endocrinopathies (58.3%) were lower with EES compared with published results with transcranial resection7–9.
The fundamental objective of radiotherapy is to deliver a therapeutic dose to the tumor target while limiting the dose to nearby normal structures. Intensity-modulated photon radiotherapy (IMRT) is a precise radiotherapy modality that tailors small beamlets of varying intensities to a complex target structure. Compared with three-dimensional conformal radiotherapy (3DCRT), IMRT offers improved dose conformality and reduced dose to adjacent normal structures. In a dosimetric study of 15 pediatric craniopharyngioma patients who underwent treatment planning for both 3DCRT and IMRT, IMRT reduced the mean dose to the cochlea from 18.2 to 13.3 Gy (p <0.001), temporal lobes from 14.3 to 7.9 Gy (p <0.001), and hippocampus from 26.8 to 17.6 Gy (p <0.001)10.
Protons from a cyclotron or synchrotron travel through tissue delivering small dose until reaching their maximum depth, where, depending on their energy, they deposit a narrow distribution of dose before stopping, producing the characteristic Bragg peak. Unlike in photon-based radiation, no “exit” dose is delivered beyond the target with proton therapy. A “spread-out” Bragg peak can be created by delivering protons across a range of energies. In a dosimetric study of 10 pediatric craniopharyngioma patients who underwent treatment planning using IMRT, three-dimensional conformal proton radiotherapy (3DCPT), and intensity-modulated proton therapy (IMPT), both 3DCPT and IMPT demonstrated a relative reduction in the integral dose to the brain stem, hippocampus, dentate gyrus, vascular structures, subventricular zone, infratentorial region, supratentorial region, and whole brain11. Such a dose reduction to these intimately located critical structures can help lessen the acute and late toxicities of radiotherapy. Investigators of a Childhood Cancer Survivor Study analyzing pediatric patients with a variety of tumors calculated a 2- to 15-fold reduction in the incidence of second malignancies when proton therapy replaces conventional photon radiotherapy12. Compared with photon therapy, proton therapy offers a better opportunity to preserve IQ scores in patients with craniopharyngioma13. In a review of 40 pediatric craniopharyngioma patients who received proton radiotherapy, the 5-year local control and overall survival rates were 100%14. Table 1 reviews the published outcomes on patients with craniopharyngioma treated with proton therapy14–22. A comparison of photon stereotactic radiotherapy and 3DCPT plans is shown in Figure 1.
| Study; number of patients | Median follow- up, years | Treatment modality | Actuarial 5-year local control rate | Acute toxicity, number of patients | Late toxicity, number of patients | Mortality (absolute number), number of patients |
|---|---|---|---|---|---|---|
| Fitzek et al.15 (2006); N = 15 | 13.1 | Surgery/biopsy + proton-photon | 93% 5-year | None, 7; nausea, 1; fatigue, 3; headaches, 4 | Visual deficits, 2; endocrinopathy, 15; learning difficulty, 1 | PD, 2; vascular complications, 1; treatment related hypothalamic syndrome, 1 |
| Luu et al.16 (2006); N = 16 | 5 | Surgery + proton or proton alone (surgery + RT, 4; recurrent after surgery, 12; re-resection + RT, 7; RT, 4) | 94%a (recurrence at 80 months, 1) | NR | Panhypopituitarism, 1; CVA, 1 with full recovery; meningioma, 1 following re-irradiation | 3 at 12, 52, and 120 months after re-resection and RT (PD, 1; sepsis, 1; MCA infarct, 1) |
| Winkfield et al.17 (2008); N = 24 | 3.7 | Surgery/biopsy + proton therapy | 100% | NR | NR | Intracranial hemorrhage at 1 year, 1 (in a child with 3 previous surgeries followed by RT) |
| Chang et al.18 (2009); N = 14 | 1.3 | Surgery/biopsy + proton therapy | 100%a | NR | Vision, stable or improved; endocrinopathy, 11 (of 11 with results) | 0 |
| Alapetite et al.19 (2012); N = 49 | 4.4 | Surgery + proton- photon, 10; surgery + proton, 39 | 90%a | NR | Altered short-term memory, social and emotional functioning and significant school difficulties in children who had RT after several surgeries. Behavioral disorder rates lower after STR + RT. | NR |
| Confer et al.20 (2012); N = 13 | 0.7 | Surgery/biopsy + proton | 85%a | Grade 2 headache, 1 | NR | 0 |
| Indelicato et al.14 (2012); N = 40 | 0.7 | Surgery/biopsy + proton therapy | 100%a | Emesis, 1; headache, 1; presyncope, 2; nausea, 9 | None to date | 0 |
| Bishop et al.21 (2014); N = 21 | 2.75 | Surgery/biopsy + proton, 15; proton alone, 4 | 92% | NR | Vasculopathy, 2; endocrinopathy, 16 | Secondary to surgically induced DI, 1 |
| Merchant et al.22 (2017); N = 94 | 2.65 | Surgery/biopsy + proton therapy | 97.8% (3-year) | NR | Preservation of academic achievement | NR |
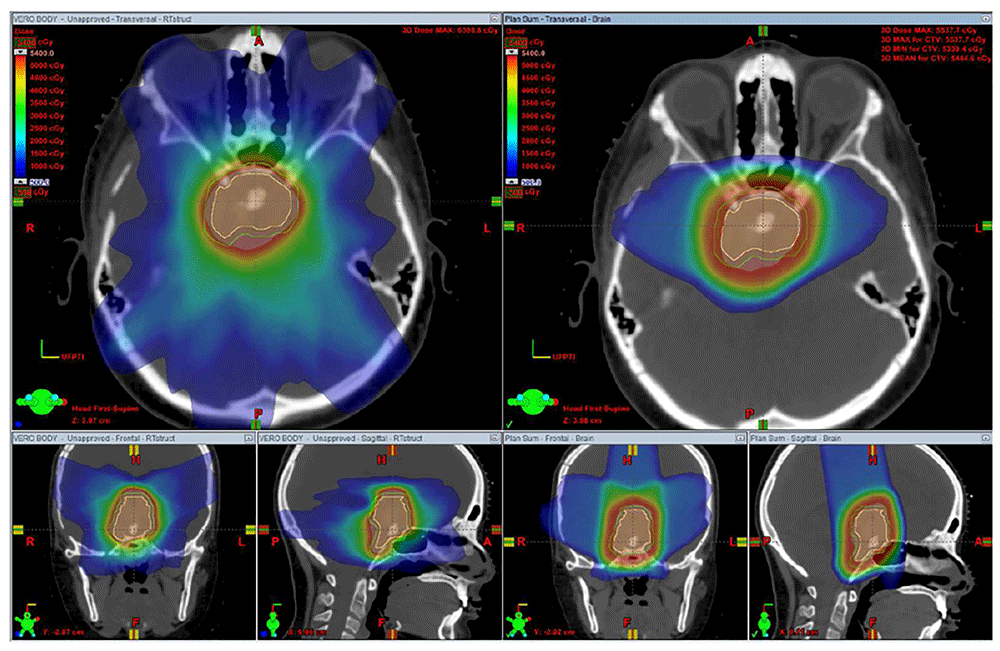
Stereotactic radiotherapy (left) offers a sharp dose fall-off, but owing to the seven-beam arrangement with both entry and exit dose, a low dose is deposited diffusely in the region of the normal brain surrounding the central tumor. Three-dimensional conformal proton therapy (right) offers a sharp dose fall-off and, with a three-beam arrangement with no exit dose, offers less dose deposition in the normal brain tissue. The proton plan offers better sparing of the supratentorial brain and orbits. The figures are original images taken in our clinic for this publication.
Spot scanning provides better proximal and distal target conformality compared with passive-scatter proton therapy by covering the target with small mono-energetic pencil beams steered by magnets. Dosimetric studies have shown that IMPT decreases the dose to normal surrounding tissue compared with double-scatter proton therapy23, as shown in Figure 2. However, spot scanning is more sensitive to changes in the volume of cystic craniopharyngiomas during treatment, which could lead to underdosing at the margins. Furthermore, most spot-scanning systems do not allow aperture-based delivery, which means that the beam penumbra may be less conformal at the lateral target.
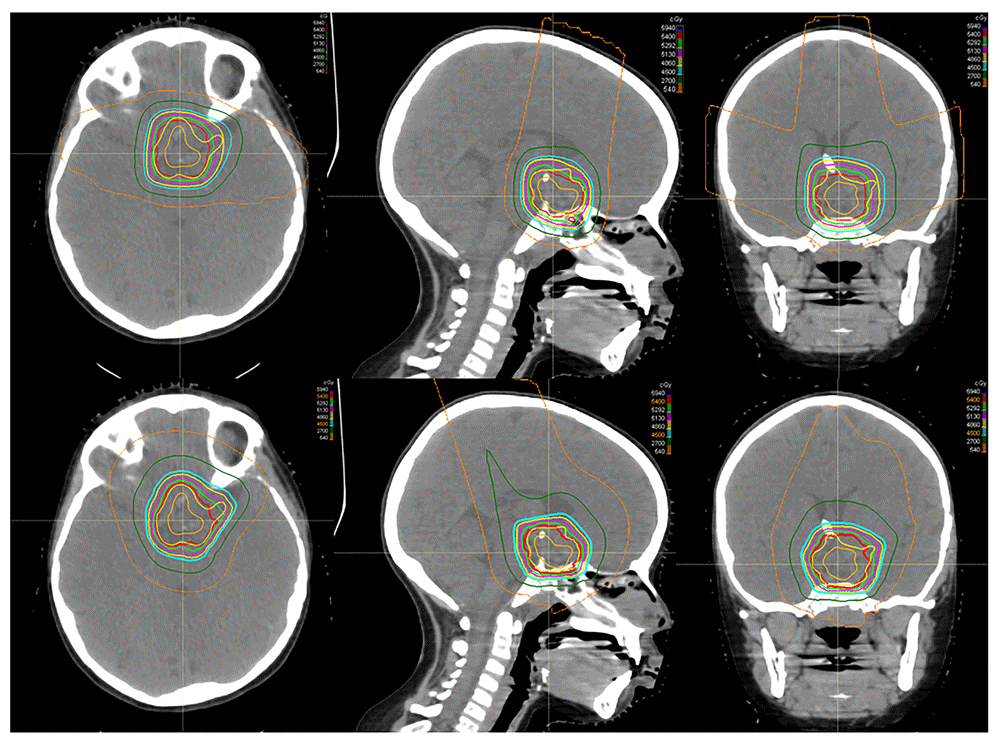
An example of the dose distribution for passive-scatter proton therapy (top) and pencil-beam scanning (bottom) in a patient with craniopharyngioma demonstrates comparable and acceptable target coverage in both plans. In this example, however, the pencil-beam modulation is used to create a more homogenous dose plan. The maximum doses to the optic chiasm are 55.6 Gy for the passive-scatter plan and 54.6 Gy for the pencil-beam scanning plan. The figures are original images taken in our clinic for this publication.
In radiation planning for all modalities, target volumes are delineated by using computed tomography simulation images fused to T1- and T2-weighted post-contrast thin-sliced (1 to 1.5 mm slice thickness) magnetic resonance imaging (MRI). The gross tumor volume (GTV) has historically been expanded by a margin of 10 mm to create the clinical target volume (CTV). However, in a prospective analysis of 88 children who received radiotherapy between 1998 and 2009, a 5 mm and a 10 mm expansion for the CTV provided comparable PFS1. A recent phase II protocol (RT2CR, NCT01419067) at the University of Florida in conjunction with St Jude Children’s Research Hospital prospectively evaluated a 5 mm CTV margin using proton therapy. Early results are promising in terms of both disease control and reduced toxicity22,24.
Improved conformality with the most recent technological advances in radiation therapy delivery increases the susceptibility of the proton dose distribution to the effects of dynamic cyst changes that occur throughout the 6 weeks of treatment. The GTV, by virtue of cyst reduction or enlargement, has been observed to change on average by 28.5% (range of −20.7% to 82%) during treatment when weekly MRIs are obtained10. Weekly MRIs during radiotherapy are used to identify these changes, and adaptive planning is necessary when changes in tumor volume may impact target coverage, as shown in Figure 3.

Pictured are the T2-weighted magnetic resonance images (MRIs) in an axial, sagittal, and coronal presentation (left to right) taken weekly during radiotherapy for craniopharyngioma. The most superior MRI was obtained for radiation planning purposes, and the red line represents the contoured gross tumor volume. The images in the second row were obtained during the first week of radiation treatment and demonstrate cyst growth. The most inferior images were obtained during the second week of radiation treatment after the cyst had been drained via the Ommaya reservoir. The figures are original images taken in our clinic for this publication.
Craniopharyngioma is a curable benign tumor treated primarily by conservative resection and radiotherapy. Reducing the late toxicities of radiotherapy remains of pivotal importance in treating craniopharyngioma. Recent technological advances in radiotherapy offer the promise of reducing side effects while maintaining high cure rates.
3DCPT, three-dimensional conformal proton therapy; 3DCRT, three-dimensional conformation radiation therapy; CTV, clinical tumor volume; EES, endonasal surgery; GTV, gross tumor volume; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated radiation therapy; MRI, magnetic resonance imaging; PFS, progression-free survival; STR, subtotal resection
The authors thank Jessica Kirwan for editing and preparing the manuscript for submission.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 11 Oct 18 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)