Keywords
α-tocopherol, antioxidant capacity, β-carotene, deodorization, olein fraction of red palm oil
This article is included in the ICTROPS 2018 collection.
α-tocopherol, antioxidant capacity, β-carotene, deodorization, olein fraction of red palm oil
The attempt to produce multivitamins locally sourced from the emulsion of the olein fraction of red palm oil (OFRP), pumpkin juice (PJ), and dragon fruit juice (DFJ) was hampered by the strong odor and aftertaste of OFRP1. The stripping of vapor components from OFRP is theoretically achievable through heating at low pressure or flowing inert gas as a carrier of the evaporated odor compounds2. However, application of heat to reduce the off-taste compounds in OFRP may result in the formation of trans-fatty acids and the occurrence of lipid oxidation. Oxidation is a major concern to edible oil quality, deteriorating its chemical, sensory and nutritional properties. The application of heat is also limited the desire to conserve the minor antioxidant components (e.g. tocopherols compounds)3.
Maintaining healthy contents of natural antioxidants and poly-unsaturated fatty acids (PUFA) are key indicators of the quality of refined and deodorized edible oils4. In this regard, natural antioxidants protect oils from hydrolysis, oxidation, polymerization, isomerization, and cyclization5. Previous deodorization efforts to reduce the off odor of red palm oil resulted in α-tocopherol being a better marker for maintaining overall antioxidant contents. An important finding was the need to maintain the critical time of the deodorization, which the destruction of tocol groups of the OFRP was in linear correlation to the deodorization time6.
Vacuum evaporation and steam-assisted deodorization are common practices in oil refinery. Applying vacuum pressures of 10-5 to 1 mbar enables heat application to reach 300°C in edible oil processing7. Steam-assisted deodorization requires good contact between the edible oils and the steam as stripping medium4. A nitrogen medium is preferable in comparison to steam medium, due to the inert property of the gas and the effectiveness of thermal breakdown of off-taste precursors in the presence of the nitrogen carrier inside the deodorization chamber8. Based on various application reports9–12, both methods are capable of deodorizing off-odor compounds in the production of better-tasting and rich-in-antioxidant OFRP.
Concerning the challenges of OFRP deodorization, this stage should be revisited and further developed to produce a higher antioxidant capacity in the final OFRP, PJ, and DFJ emulsion products. This study aims to optimize the vacuum evaporation and nitrogen-assisted deodorization method of OFRP, observed using the levels of β-carotene, α-tocopherol, percentage inhibition of ABTS reduction, and ferric reducing antioxidant power (FRAP) activity.
The crude palm oil (CPO) was obtained from PT Rea Kaltim, Indonesia. The olein fraction of red palm oil (OFRP) was prepared from CPO which had been tested for free fatty acid (FFA) levels and then carried out neutralization and gum removal by adding 100 ml warm water (80–90°C). Shuffling of the sample was carried out for 1 minute in a separating funnel before removal of residual water. About 400 μl of 10% NaOH (Emsure® Merck Cat no. 106498, USA) was added. Rinsing with 50 ml of warm water was carried out repeatedly until two phases of mixture were formed. The OFRP was produced with yields ranging from 60 to 70% of CPO (Rahmadi et al., 2015).
Deodorization of the OFRP was prepared by two methods, namely vacuum and nitrogen-assisted evaporation. The liquid fraction was deodorized using a rotary evaporator (Büchi R-200, Switzerland) at a combination of temperature and pressure: (1) olein fraction processed with vacuum evaporation 1 (OFV1): 90°C, 80±5 mmHg, (2) OFV2: 100°C, 80±5 mmHg, (3) OFV3: 90°C, 100±5 mmHg, (4) OFV4: 100°C, 100±5 mmHg, and speed of 60 RPM for 1 hour. The OFVs that has been obtained was then stored in a closed container in the refrigerator (4±2°C) for further processing. Deodorization of the olein fraction by the nitrogen-assisted method was prepared by addition of 100 ml OFRP in a closed container. The sample had added to it 2% (w/v) pharmaceutical-grade activated carbon (Norit, Japan), then pure-grade nitrogen gas (Samator Gas, Indonesia) was flowed using a valve for 15 minutes (OFN1) and 30 minutes (OFN2) at a temperature of 85±3ºC, and a flow rate of 1 l/minute.
Preparation of pumpkin and red dragon fruit juice was carried out according to previously described method13. A total of 1 kg of each peeled fruit was cut into pieces of approximately 3–5 cm3 and washed using clean water. Then, the pieces of fruit were mashed using a commercial juicer. After that, the juice was put separately in glass bottles and pasteurized at 80°C for 10 minutes, then filtered using a clean filter cloth. The filtered fruit juices were then stored at 4±2°C before being utilized.
A total of 30 ml of the deodorized OFRP was added to 70 ml pumpkin juice, giving a total volume of 100 ml. Food grade carboxymethylcellulose, xhantan gum, and cinnamon powder were added at concentrations of 2% (w/v), 2% (w/v), and 0.5% (w/v), respectively. After homogenization, the product samples had added to them 75% (v/v) of red dragon fruit juice in warm water (80–90°C) to a volume of 400 ml, then fructose syrup sweetener, citric acid and raspberry flavoring were added at 10% (v/v) and 0.25% (w/v), and 0.7% (v/v), respectively. The mixture was homogenized with a blender at low speed for 3 minutes. The samples were filtered and placed in a dark glass bottle. The bottles containing the emulsion product were then pasteurized at 70°C for 15 minutes.
FFA levels, peroxide values (PV), and acidic values (AV) were determined as previously described14.
Carotenoids were measured via measurement of β-carotene by modification of the Palm Oil Research Institute (PORIM) method15, with changes in wavelength 446 to 443 nm (Rayleigh model UV 2601, China) and solvent changed from n-hexane to absolute methanol (Fulltime cat. 6501-04, China). Quantitative determination of β-carotene levels in samples was obtained based on calibration curves with β-carotene standards (Sigma-Aldrich cat. no. C9750-10G, UK).
The determination of α-tocopherol was accomplished by interpolating the absorbance of the sample with a standard calibration curve of α-tocopherol at a wavelength of 291 nm (Rayleigh model UV 2601, China). The α-tocopherol standard (Sigma-Aldrich cat. no. T3251-25G, UK) was prepared in absolute ethanol (Smartlab cat. no. A1035, Indonesia) with various concentrations of 50. 75. 100. 125 and 150 mg/L in 10 mL of absolute ethanol. Blank sample was prepared without containing the active substance of α-tocopherol.
Total betacyanin quantification was carried out as described16, and calculated using established equation17. A total of 1 g of sample was macerated with 5% HCl solution (Merck cat. no. 109063, USA) (1:10) in a dark bottle, then stored at 4±2°C for 24 hours. The mixture was vacuum-filtered with Whattman's filter paper no. 4. Next, 5 ml filtrate was diluted with 95% ethanol solution: 1.5 N HCl (85:15) to 10 ml. The absorbance was measured at a wavelength of 535 nm (Rayleigh model UV2601, China).
ABTS. For the preparation of antioxidant measurement by the ABTS method, solution A was prepared by dissolving 7.1015 mg of 2.2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) (Sigma-Aldrich cat. no. A1888-1G, UK) in 5 ml distilled H2O, and solution B was prepared by dissolving 3.500 mg K2S2O8 (Sigma-Aldrich cat. no. 216224-100G, UK) in 5 ml of distilled H2O. Solution A and B were separately incubated in dark bottles for 12 hours. After incubation, solution A and B were mixed in a dark room and added with absolute ethanol to produce 25 ml of the final solution. Measurement of absorption of ABTS blank solution was carried out at a wavelength of 750 nm (Eppendorf Single Beam BioSpectrometer®, Germany). Measurement of free radical-binding activity was carried out by mixing the emulsion sample with ABTS solution at a concentration of 100 ppm. The sample was measured at maximum absorbance wavelength. butylated hydroxytoluene (BHT) was used as a standard (Sigma-Aldrich cat. no. W218405, UK). The percentage of antioxidant activity was determined based on the calculation of the difference between the blank and the sample absorbance divided by the blank absorbance.
FRAP. FRAP reagent consists of 0.1 M acetate buffer (pH 3.6) (Merck cat. no. 107827, USA), 10 mM 2.4.6-tripydyl-s-triazine (TPTZ) (Sigma-Aldrich cat. no. 93285-5G, UK), and 20 mM FeCl3•6H2O (Sigma-Aldrich cat. no. 157740-100G, UK) in a ratio of 10:1:1 in 40 mM of HCl. Standard solutions of FeSO4•7H2O (Sigma-Aldrich cat. no. 215422, UK) were prepared at concentrations of 10, 20, 30, 40 and 50 µmol/l. The maximum absorbance of FeSO4•7H2O was determined, which was at wavelength of 594-597 nm (Eppendorf Single Beam BioSpectrometer®, Germany). A total of 0.1 ml emulsion sample was prepared and added to 3 ml FRAP reagent in a test tube. The absorbance of the mixture was then read at 574 nm. Quantitative determination of total antioxidants in the sample was obtained based on calibration curve of FeSO4•7H2O.
FFA indicates the palatability acceptance of the OFRP and its emulsion-derived products; a higher presence of FFA contents results in a more pronounced sharp taste. FFA standard for fresh palm oil is less than 2%, while maximum FFA content for processed palm oil is set to 5%18. The vacuum evaporation of OFRP produced a higher concentration of FFA in comparison to nitrogen-assisted deodorization. However, the final products had less than 2% of FFA (Figure 1).
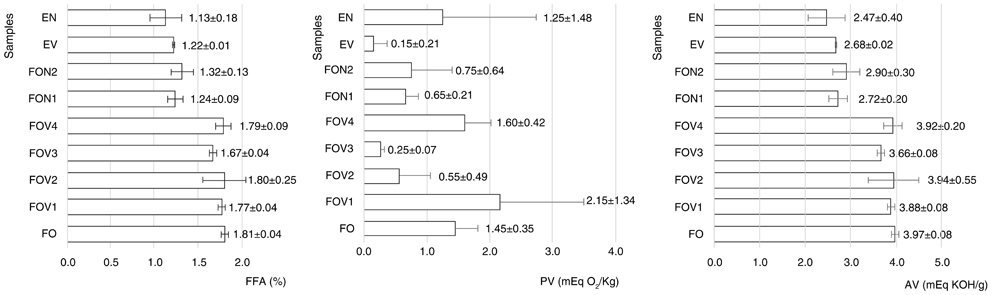
PV indicates the presence of hydrogen peroxide in edible oils. The standards for the maximum PV in edible oils are determined based on the product and the processing technology used19. For example, the standard PV for virgin olive oil is not more than 20 mEq O2/Kg, while for processed olive oil is not more than 10 mEq O2/Kg20. Initial OFRP had 1.45±0.35 mEq O2/Kg. Neither vacuum-evaporated nor nitrogen-assisted deodorized OFRP had PV of more than 3 mEq O2/Kg. The final emulsion products had PV of less than 2 mEq O2/Kg (Figure 1).
OFRP and the derived emulsion products had an AV of less than 4 mEq KOH/g (Figure 1). Pure palm oil had AV on average of 10 mEq KOH/g. in acidic environment21. Crude Palm Oil (CPO) produced from small scale palm oil mills had an average AV of 18 mEq KOH/g22,23. From the two deodorization approach, in terms of values of FFA, PV, and AV, nitrogen-assisted deodorized OFRP emerged as the better deodorization process.
The PORIM method15 allows for quick determination of the β-carotene content in CPO. Based on the procedure and to ensure high repeatability, it is necessary to recheck the peak wavelength of β-carotene standard, which was at 443 nm, and to generate a β-carotene standard curve (Figure 2a). The contents of β-carotene in OFRP, PJ, DFJ and derived products are given in Table 1. The relative contents of β-carotene to OFRP were highlighted in Figure 2b. As expected, DFJ had the lowest percentage of β-carotene in comparison to OFRP, while the content of β-carotene in PJ was 60% of the content in OFRP. The β-carotene contents for the emulsion made using vacuum-deodorized OFRP (EV) was 271.7±2.4 ppm, while 259.9±1.4 ppm of β-carotene was found in the emulsion made of nitrogen-assisted deodorized OFRP (EN).
OFRP, olein fraction of red palm oil; DFJ, dragon fruit juice; PJ, pumpkin juice; OFV1, OFRP treated at 90°C, 80±5 mmHg, 60 RPM, for 1 hour; OFV2, OFRP treated at 90°C, 100±5 mmHg, 60 RPM, for 1 hour; OFV3, OFRP treated at 100°C, 80±5 mmHg, 60 RPM, for 1 hour; OFV4, OFRP treated at 100°C, 100±5 mmHg, 60 RPM, for 1 hour; OFN1, 85±3 °C, flow rate of 1 l/minute of nitrogen for 15 minutes; OFN2, 85±3°C, flow rate of 1 L/minute of nitrogen for 30 minutes; EV, emulsion made of OFV3. PJ and DFJ; EN, emulsion made of OFN1. PJ and DFJ; n.t., not tested.
The use of higher temperatures in vacuum evaporation (OFV2 and OFV4) slightly reduced β-carotene content in OFRP. A temperature increase without an increase in head pressure resulted in a lower accumulation of β-carotene, as observed in OFV4 vs OFV2 and OFV3 vs OFV1. This phenomenon is in line with that previously reported24, stating that a temperature increase while reducing head pressure resulted in lower efficiency of soybean oil neutralization and distillation. Nitrogen-assisted deodorization helped to concentrate β-carotene content in OFRP. The emulsion products contained around 50% of β-carotene in OFRP. Nitrogen was used as a stripping medium substitute for steam, owing to its inert and easy-to-remove properties of non-reacting. This highlights the possibility of neutralizing distillation by stripping with nitrogen in physical refining to obtain refined oils within specified end product's acidity and flavour24.
To provide a repeatable measurement of the content of α-tocopherol, peak wavelength of α-tocopherol standard was rechecked and occurred at 291 nm and α-tocopherol standard curve was produced as in Figure 3a. Table 1 displays the α-tocopherol content in OFRP, PJ, DFJ and derived products, while Figure 3b highlights the relative contents of α-tocopherol to OFRP. All three sources (OFRP, DFJ and PJ) contained a reasonable concentration of α-tocopherol, (70.61±0.59, 37.02±0.33 and 32.95±0.04 ppm, respectively). The final emulsion products contained between 36.36±0.20 and 39.12±0.20 ppm of α-tocopherol.
Previously, we reported that processing time was critical to maintain α-tocopherol levels in OFRP deodorization6. While the deodorization time was fixed at 1 hour, a combination of temperature and vacuum pressure (OFV1 to OFV4) had slight effect in reducing the content of α-tocopherol in OFRP The phenomenon was similar to that previously reported24, which found that a 10°C temperature increase resulted in slightly reduced α-tocopherol content in soybean oil neutralization and distillation. The best treatment for vacuum deodorization was at 100 °C and 100±5 mmHg (OFV3).
The presence of betacyanin in dragon fruit has led to the use of the color for dye in various applications, from food to solar cells16,25. DFJ juice contains 2.18 ppm of betacyanin, while the contents of betacyanin in the emulsion products were 22–26% of the content in DFJ (Figure 4). This indicated that the function of DFJ was to provide pleasant color while masking the after taste of OFRP in the final emulsion product1.
The antioxidant activity of OFRP, PJ, DFJ, and the derived emulsion products were estimated with ABTS and FRAP assays (Figure 5). The two methods were suitable to measure antioxidant activity in oil based products26. Products containing high concentrations of carotenes, xanthophylls and tocopherols are expected to have moderate-to-strong antioxidant potency, comparable to BHT. In comparison to BHT, the emulsion products had 22.93±0.10 to 32.11±0.04% of free-radical-scavenging activity, as measured by ABTS method. There was no significant difference of percentages of antioxidant activity of OFRP, PJ, DFJ, and the derived emulsion products against the BHT standard. Based on the ABTS and FRAP assays, it was concluded that OFRP, PJ, DFJ, and the derived emulsion products exhibited moderate-to-strong antioxidant activity.
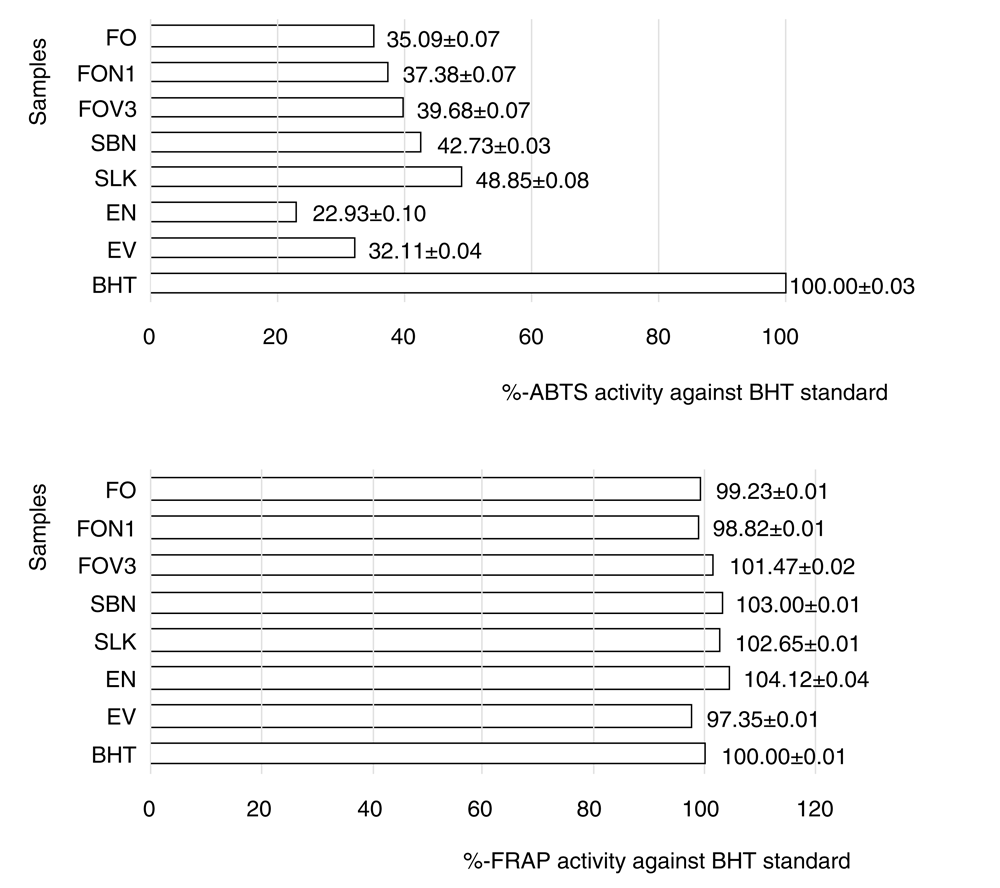
While acting as a putative chain-breaking antioxidant, β-carotene was capable of scavenging peroxyl radicals27. Tocopherols are strong antioxidants, but their activity depends on the isomers, reactivity of the tocopheryl radical, surrounding temperature, and the type and viscosity of emulsion28. Oil-based products may contain secondary antioxidants, due to their greater ability to reduce lipid oxidation than acting as free radical scavengers26.
The vacuum evaporation of OFRP produced slightly higher FFA and AV in comparison to nitrogen-assisted deodorization, while the final products had less than 2% of FFA, less than 3 mEq O2/Kg of PV, and less than 4 mEq KOH/g of AV. The β-carotene contents for the emulsion containing vacuum-deodorized OFRP was at 259.9±1.4 and the nitrogen-assisted deodorized OFRP was 271.7±2.4 ppm; these values were 51 and 53% of the β-carotene values in the initial OFRP, respectively. The use of higher temperatures in vacuum evaporation slightly reduced the β-carotene content in OFRP, while nitrogen-assisted deodorization concentrated β-carotene content in OFRP. All three sources (OFRP, DFJ, PJ) contained reasonable concentrations of α-tocopherol (70.61±0.59, 37.02±0.33, and 32.95±0.04 ppm, respectively), while the final emulsion products contained 36.36±0.20 to 39.12±0.20 ppm of α-tocopherol. The main purpose of DFJ was to provide pleasant color while masking the after taste of OFRP in the final emulsion product. OFRP, PJ, DFJ, and the derived emulsion products exhibited moderate-to-strong antioxidant activity. The best vacuum evaporation condition was at 100°C, 80±5 mmHg, 60 RPM, for 1 hour, while the best nitrogen-assisted conditions were 85±3°C with flow rate of 1 l/minute of nitrogen for 15 minutes.
Dataset 1. All data on the properties of the emulsions produced in the current study. Included in labelled files are all raw data for each variable measured in this study, alongside the processed data. DOI: https://doi.org/10.5256/f1000research.16545.d22173829.
Authors thank the Indonesian Ministry of Research, Technology, and Higher Education, which funded this research in 2018.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: medicinal chemistry, Natural products chemistry and engineering, food and environmental biotechnology, microbial biotechnology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: food process engineering, enzymatic membrane reactor, bioprocess engineering
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 31 Oct 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)