Keywords
Chikungunya, High content screening, drug discovery, antivirals
This article is included in the Neglected Tropical Diseases collection.
Chikungunya, High content screening, drug discovery, antivirals
Chikungunya virus (CHIKV) is an arthropod-borne virus that belongs to the Alphavirus genus of the Togaviridae family. Alphaviruses are positive-sense, single-stranded RNA viruses that can produce severe encephalitis, such as in the infections caused by Ross River virus (RRV), Western- (WEE), Eastern- (EEE) and Venezuelan-equine encephalitis (VEE) virus. Alphaviruses can also be arthritogenic, such as in the case of CHIKV, Mayaro virus (MAYV), and O’nyong’nyong virus (ONNV)1. CHIKV was responsible for several recent (re)emerging outbreaks in humans2–4. Nowadays, approximately one billion people around the globe, especially in the tropics, are estimated to live in risk areas of CHIKV outbreaks4,5. In the Americas, CHIKV was first detected in 2013, in St. Martin, an island in the Caribbean, and quickly spread to other countries, including Brazil6. CHIKV produces an acute disease with high fever, headache, nausea, vomiting and conjunctivitis. Patients also develop severe joint pain, which eventually evolves into an arthritogenic syndrome that can last from weeks to years7. Recently, CHIKV infection has also been associated with neurological complications8. There are no antiviral drugs or vaccines available for CHIKV, and the supportive care treatment aims at reducing symptoms and include analgesics, anti-inflammatory and antipyretic drugs.
Some anti-CHIKV molecules have been discovered as a result of antiviral screening campaigns, such as a harringtonine, a plant alkaloid that reduced CHIKV replication by interfering with protein translation in vitro9; D-N4-hydroxycytidine (NHC), a nucleoside analogue, that inhibits RNA synthesis by targeting replication complex10; and barberine, abamectin and ivermectin, which all also reduce viral RNA synthesis11. Most assays were based on replicon systems, a classic way to evaluate drugs that interfere with the viral replication phase, but which cannot account for drugs that might inhibit other steps of the viral cycle, such as cell entry or virion assembly and release. Thus, alternative assays that deploy infectious viral particles, such as those that are based on measurement of cellular infection by high-content screening (HCS)12,13, enable the investigation of compounds that may interact with different stages of infection and lead to the to discover of new classes of antivirals.
Huh-7 hepatocellular carcinoma cells were cultivated in DMEM F-12 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (Life Technologies), 100 units/ml Penicillin (GIBCO) and 100 µg/ml Streptomycin (GIBCO) at 37°C, 5% CO2. The Vero cell line derived from the kidney of an African green monkey were cultivated in DMEM high glucose (GIBCO) supplemented with 10% FBS (Life Technologies), 100 U/ml penicillin (GIBCO) and 100 μg/ml streptomycin (GIBCO) at 37°C, 5% CO2. Cells were kindly provided by Professor Amilcar Tanuri (UFRJ-Brazil).
The CHIKV 181/25 vaccine strain was used in the present study14. The strain was obtained from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) of the University of Texas Medical Branch. The viral stock was propagated in Vero cells. Supernatant of infected tissue cultures was harvested and titrated by plaque assay15. Briefly, Vero cells were seeded in 24-well plate and incubated at 37°C, 5% CO2 for 24 hours. Virus suspension was diluted 10-fold in DEMEN high glucose, and 0.2 µl from each virus dilution was added to infect Vero cells. After 1 hour in 37°C, 5% CO2, inoculum was removed and cells were washed twice with Dulbecco’s Phosphate-buffer saline (DPBS pH 7.4, Sigma-Aldrich). An overlay was added with High-glucose DMEM (GIBCO), 10% FBS (Life Technologies) and 3.5% carboxymethylcellulose (CMC, Sigma-Aldrich) prepared in distillated water. At 3 days after, the overlay was removed. Then, cells were fixed with 4% paraformaldehyde (PFA) diluted in DPBS and stained with 0.5% crystal violet to enable plaque visualization and counting. Virus titers were expressed as plaque forming units (PFU) per milliliter.
Mouse hyperimmune sera was obtained from previously prepared stocks16. Briefly, to prepare these stocks mice (Mus musculus) received 4 weekly inoculations of 0.2 ml of brain macerate suspensions from newborn mice infected with CHIKV in PBS, by the intraperitoneal route. At 5 days after the last immunization the animals were anesthetized and underwent intracardiac puncture for blood collection. CHIKV-MHS was obtained from this blood.
The compounds 6-azauridine (CAS #54251), interferon α2A (CAS #H6041), bafilomycin A1 (CAS # 88899552), chloroquine (CAS #50635), 5-fluorouracil (CAS #51218) and the Library of Pharmacologically Active Compounds (LOPAC), containing 1,280 compounds, were purchased from Sigma-Aldrich. Sofosbuvir and daclatasvir were kindly provided by Microbiológica Química e Farmacêutica (Brazil) and ledispavir was donated by MedChemExpress (USA).
Huh-7 cells were seeded in black polystyrene 384-well assay plates (Greiner Bio-One) at 3,000 cells/well in 40 µl DMEM-F12 supplemented with 10% FBS and incubated overnight. Cells were infected with 10 µl of inoculum of CHIKV 181/25 at different multiplicities of infection (MOIs) of 0.5, 0.05 and 0.01. Plates were fixed at different periods of time (36, 48 and 72 hours) and submitted to the immunofluorescence assay (described below) and images are acquired using an InCell Analyzer 2200 (GE Life Sciences).
A library stock plate containing the aforementioned compounds at 2 mM in DMSO was used to prepare the intermediate plate by a 16.6-fold dilution in DPBS, to a concentration of 60 µM and 3% DMSO. Then, 10 µl of the intermediate plate content was transferred onto the cell-containing plate. The final concentration of library compounds in the assay plate was 10 µM, with 0.5% DMSO. Controls were placed in lateral columns in all plates. Positive controls were infected cells treated with 50 µM of 6-azauridine as well as non-infected cells treated with vehicle (0.5% DMSO in DPBS). Negative controls were infected cells treated with vehicle. Cells were infected by 10 µl of CHIKV 181/25 at MOI 0.05. Plates were incubated for 48 h at 37°C, 5% CO2 under humidified atmosphere, and then fixed with 4% (w/v) PFA for 15 min at room temperature and washed twice with DPBS. Then, plates were incubated with CHIKV-MHS (mouse hyperimmune sera) diluted 1:1500 (v/v) prepared in blocking buffer (DPBS containing 5% FBS) for 30 min. Each plate was washed twice with DPBS, followed by incubation at room temperature for 30 min with the AlexaFluor488-conjugated goat anti-mouse IgG (Cat No. A-11001, Thermo-Scientific) diluted 1:2000 (v/v), and 5 µg/ml of 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) in DPBS. Each plate was washed twice with DPBS. All plates were filled up with 50 µl of PBS/well.
Images were acquired using a confocal microscope High-Content System InCell Analyzer 2200 (GE Life Sciences) and processed by InCell Investigator v.1.6.1 software (GE, USA). Four different images were acquired from each well at x20 magnification. Automated image analysis was performed cell-by-cell through a defined mask based on fluorescence signal measurement and cell morphology. Cell segmentation parameters defined the analysis performed by the Investigator software. The nuclei were segmented from the DAPI staining images and each nucleus was determined as a minimum area of 50 µM. Total cells were filtered from the AlexaFlour488 channel and were defined as minimum area of 100 µM. The mean AlexaFluor488 florescence signal of infected cells were defined from the cytoplasm mask, with values based on means signal from fluorescence of wells from infected cells showing a six-times higher value compared to the mean of Alexa488 fluorescence signal of non-infected cells. Images were treated using image analysis program ImageJ v1.51 to set up colors and merge image channels to final visualization. The validation of screening was conducted in two independent experiment.
Infection ratio (IR) was defined as the ratio between (i) the total number of infected cells, and (ii) the total number of cells. Data were normalized with the negative (DMSO-treated, infected cells) and positive (infected cells treated with 50 µM 6-azauridine) controls. Normalized activity was calculated as described by Pascoalino et al.12. Cell survival was expressed as the percentage of the total cell number from test sample divided by the average total cell number from the positive control wells: Cell number test sample/Avg. cell number of positive control) × 100. Normalized activity and cell survival values were processed with the GraphPad Prism software version 7. Representative graphs of sample distribution were obtained by plot data at TIBCO Spotfire 7.0 software. Plates were also submitted to quality control measurement of Z’-factor as described by Zhang et al.17.
For dose-response curves, drugs were prepared as described12. The initial test concentrations were 100 nM for IFN-α2A, 50 nM for bafilomycin, 120 μM for chloroquine and mycophenolic acid, and 100 μM for sofosbuvir, daclatasvir, ledispavir and 5-fluorouracil. Dose response assay were calculated based on percentage of normalized activity and cells survival for each concentration tested. Data were plotted with GraphPad Prism software version 7. The sigmodal dose-response curve (variable slope) function were used to calculate the effective concentration that inhibited 50% of infection (EC50), and the concentration of compound that presented a 50% reduction in cell number in comparison to the controls (CC50). The ratio between CC50 and EC50 determines the selective index (SI).
Two-way analysis of variance (ANOVA) with Sidak’s test, a multiple comparison test, was conducted to calculate statistical significance (P < 0.05) of cell numbers from non-infected and infected CHIKV 181/25 at different multiplicity of infection and incubation time experiment. The coefficient of determination (R2) test were using to determinate statically coefficient of variation between screening replicates from normalize activity data. All data were plotted using GraphPad Prism Software version 7.
A high-content screening assay was developed to evaluate compounds activity against CHIKV infection in vitro. The first step consisted on defining the cell model to support viral infection. A range of cell lines has been reported as being susceptible to CHIKV infection, such as Vero, human fetal lung fibroblast (MRC-5), baby hamster kidney (BHK), human embryonic kidney 293 (HEK-239T) and Huh-718,19. The Huh-7 cell line was selected as it has desirable features for high content imaging, such as adherent monolayer growth, and is human cell line, meaning it is a more representative in vitro model than would be cells of another species. The second step was determining the optimal multiplicity of infection (MOI) and the necessary period of time for the efficient viral infection in 384-well plates. Cells were infected at three different MOI (0.5, 0.05 and 0.01) and incubated for different periods of time (36, 48 and 72 hours). The total cell number and the IR were determined. When cells were plated and infected concomitantly, even the lowest MOI tested showed high cytopathic effect (data not shown). Thus, cells were plated 24 h before infection (Figure 1A). For the highest MOI, CHIKV infection decreased cell number by 70% at 48 hours and by almost 100% at 72 hours compared with non-infected cells. There was no significant difference in cell number between non-infected cells and infected cells for both 0.05 and 0.01 MOIs at 36 hours. Compared to non-infected cells, a decrease in cell number by 42% and 22% at MOIs 0.05 and 0.01, respectively, was observed at 48 hours (Figure 1A). After 36 hours of incubation, the IR showed an association with the MOI: 0.95±0.04 (MOI 0.5), 0.40±0.19 (MOI 0.05) and 0.12±0.20 (MOI 0.01) (Figure 1A), demonstrating that the assay endpoint was within the dynamic range of the infection. For 48 hours of incubation, the IR reached 0.99 for all MOIs, but the lowest MOI gave high variation in IR between replicate wells. Therefore, with the aim of testing drugs, a 0.05 MOI at 48 hours of incubation was selected for further experiments to achieve the longer time of exposure to drug treatment possible under these conditions, a high ratio of infection with minor variability, and a cell survival rate greater than 50% (compared with non-infected) (Figure 1A). Figure 1B describes the established general scheme of CHIKV high-content assay.
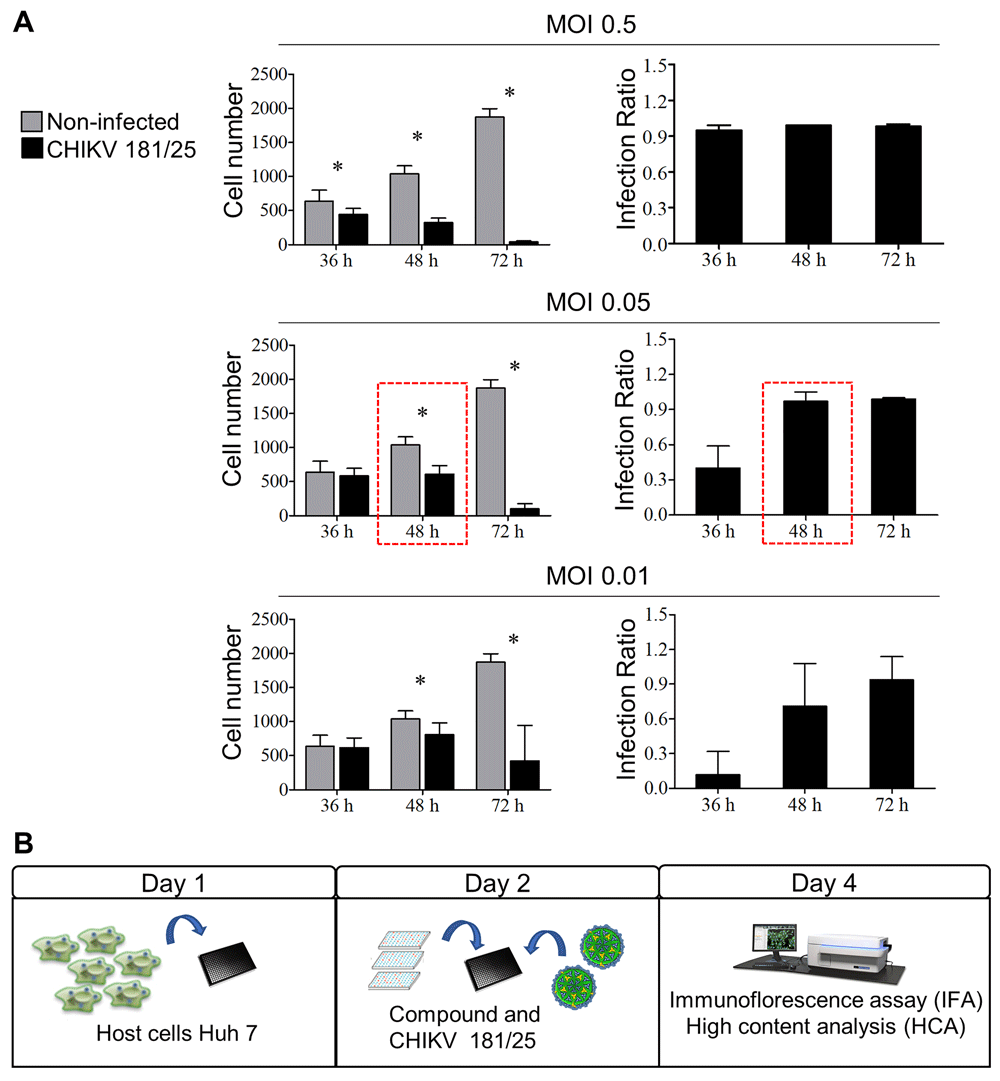
(A) Huh-7 cells were infected with different MOIs (0.5, 0.05 and 0.01) of CHIKV. The cell number and the infection ratio (defined as the ratio between total cell number and number of infected cells per sample), were evaluated after 36, 48 and 72 hours of infection. Error bars represent the standard deviation of 48 wells. Quantification of total cell number was comparable between non-infected and CHIKV 181/25-infected cells (p < 0.05). (B) General scheme of CHIKV high content assay. On day 1, Huh-7 cells were plated onto 384-well plates at 3000 cells/well. Then, after 24 hours (day 2), compounds were added, followed by addition of virus diluted at MOI 0.05. The plates were incubated for 48 hours up to immunofluorescence assay and high content analysis on day 4.
To validate the assay, high-content screening was run using a commercial library of compounds. Cell infection was determined by indirect CHIKV immunofluorescence detection. Figure 2A shows a raw image and software segmentation analysis of the same image. The 6-azauridine compound was previously reported to have activity against CHIKV20, and was chosen as the reference compound in this assay. The activity of 6-azauridine was assessed using a dose-response curve (Figure 2B). The EC50 of 0.65 µM 6-azauridine and EC100 of 50 µM 6-azauridine were determined against CHIKV. In order to validate the assay reproducibility and robustness, a commercial library composed of 1,280 compounds was tested at a single concentration (10 µM). A good window between positive and negative controls was observed (Figure 2C). As a result, the mean for all plates Z’-factor values were 0.86±0.09, indicating that the established assay is reliable. Additionally, there was a high correlation coefficient between runs (Coefficient of determination R2: 0.86), which was determined using normalized activity of each single well between the first (R1) and the second (R2) screens, including compounds and controls (Figure 2D).
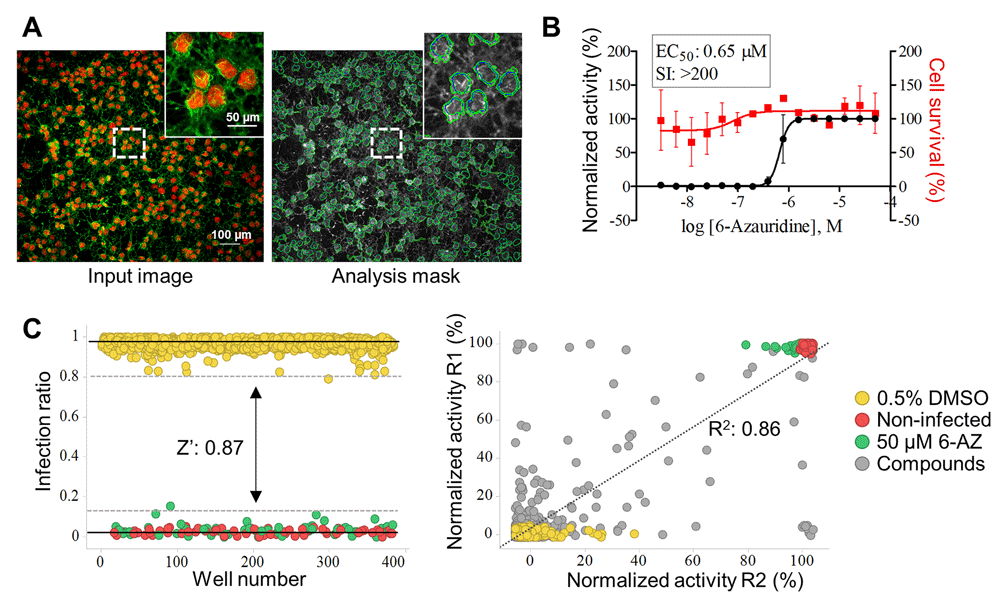
(A) Interface of image processing and analysis. (B) Dose-response curve for reference compound 6-azauridine. Left y-axis: Normalized activity values (black squares and curves); Right y-axis: Cell survival values in percentage (red dots and curves); x-axis: Log of molar compound concentrations. Data points are means, and error bars represent standard deviations from two independent experiments. (C) Left graphic: Representative scatter plot of the infection ratio showing controls separation. Dots represent each single tested well and colors represent different treatments, where: 0.5% DMSO negative control (yellow); non-infected cells (red); 50 µM 6-azauridine positive control (green). Z’-factor value of 0.87 was obtained from total data controls from two independent runs for screening validation, between inter-replicates and intra-replicates plates. The continuous line represents the mean of each control, the dotted line represents 3 standard deviations from the mean of the negative and positive controls. Right graphic: Data correlation (normalized activity) from two screening runs of a small library; dots represent each single tested well and colors represent different treatments, where: 0.5% DMSO negative control (yellow); non-infected cells (red); 50 µM 6-azauridine positive control (green); and tested compounds (gray). The coefficient of determination of R square (R2 0.86) was calculated using GraphPad Prism software.
A set of compounds with known antiviral activity were evaluated against CHIKV. A total of 9 compounds were tested in dose-response curves. Figure 3 lists the name, molecular structures and dose-response curve plots for all compounds. Interferon α2A (IFN-α2A) and mycophenolic acid have reported activity against CHIKV19,20. The HCS assay confirmed their reported activity, demonstrated by EC50 values of 0.7 nM and 0.8 μM and high SI of >14 and 8.25, for IFN-α2A and mycophenolic acid, respectively. In order to evaluate compounds with previously reported activity against CHIKV, bafilomycin A121 and chloroquine22 were tested, giving an EC50 of 0.01 μM and 21 μM, respectively; however, they were cytotoxic in Huh-7 cells, with low SI values (5 and 0.3, respectively). The antiviral activity of daclatasvir, an anti-hepatitis C virus (HCV) drug23, against CHIKV was associated with high cytotoxicity, and it had a low SI value of 1.3. Ledispavir, also an anti-HCV compound24, and 5-fluorouracil, which has reported activity against ZIKV12, did not present anti-CHIKV activity in the HCS assay. Sofosbuvir is an FDA-approved compound against HCV, and has been recently described as an active compound against other flaviviruses, including dengue and Zika25,26. Sofosbuvir demonstrated dose-dependent activity against CHIKV (EC50, 11 μM) with no cytotoxicity in Huh-7 cells. Representative images of sofosbuvir activity, alongside 6-azauridine, are displayed in Figure 4.
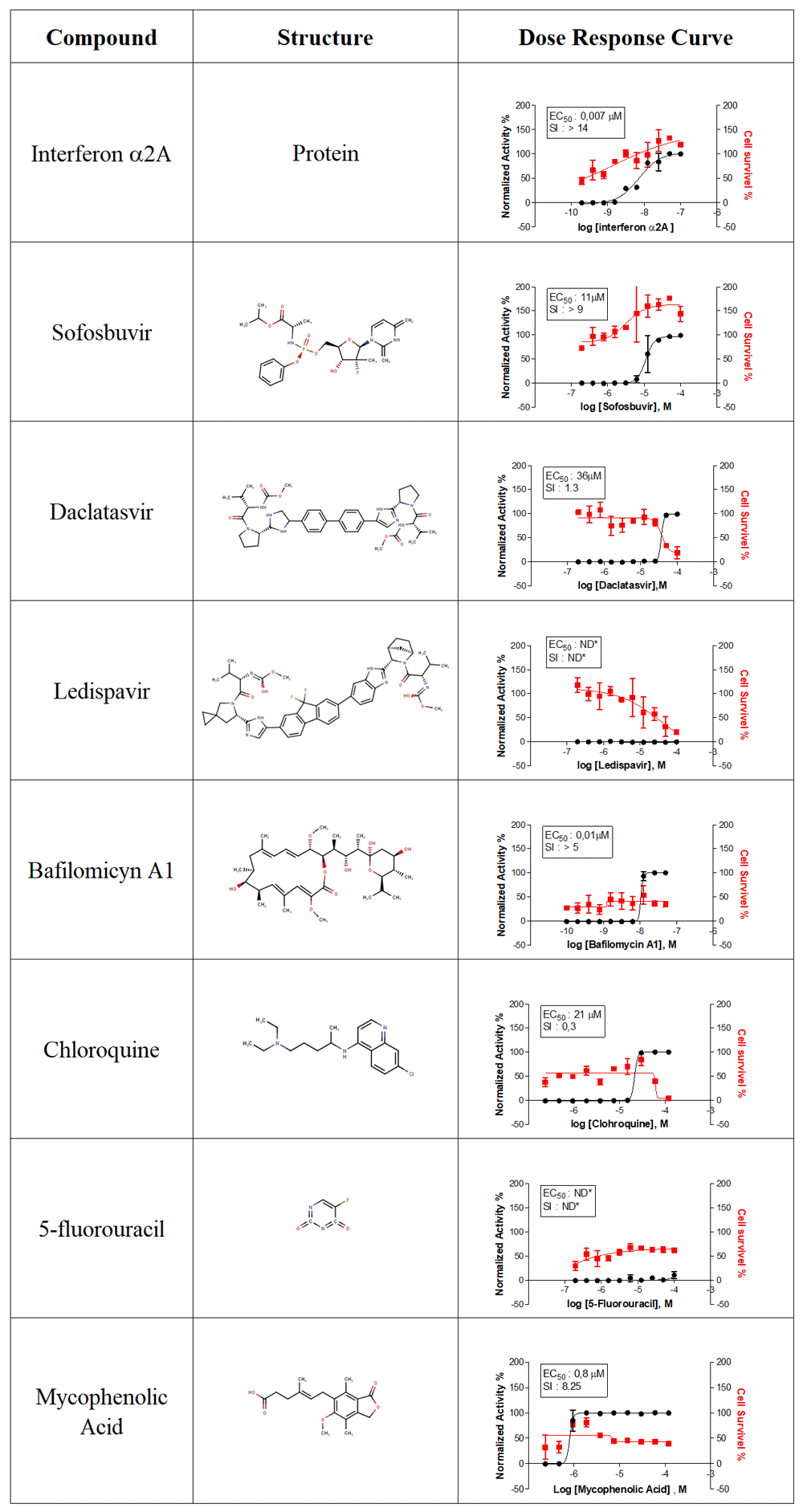
Dose response curves of interferon α2A, sofosbuvir, daclatasvir, ledispavir, bafilomycin A1, chloroquine, 5-fluorouracil and mycophenolic acid as anti-CHIKV activity (black) or the effect on Huh-7 survival (Red). Values of EC50 means effective concentration of 50% infection inhibition, and SI means selective index (SI) based on CC50 concentration of compounds of 50% cytotoxicity (not showed). ND, non-determined values.
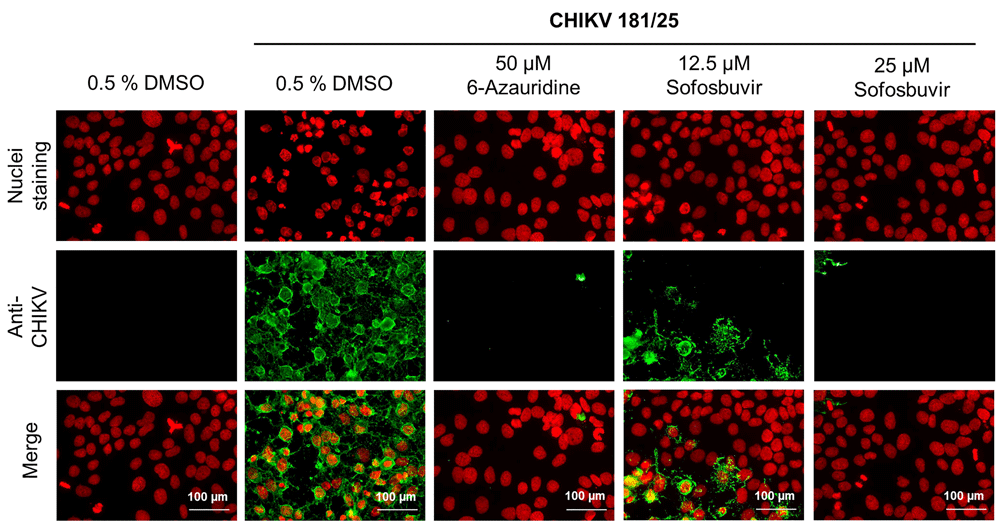
Panel of representative images. First line of images shows nuclei staining with DAPI. Second line of images shows immunofluorescence against CHIKV (AlexaFluor488). Third line of images is the merge between the lines one and two. First column displays non-infected cells, followed by infected cells treated with 0.5% DMSO, 50 µM 6-azauridine, 12.5 µM sofosbuvir and 20 µM sofosbuvir.
Currently, most assays available for drug screening against CHIKV are based on cell viability methodologies, which evaluate the compounds capacity to prevent cell lysis27–30. Such approaches have the advantage of being of lower in cost and higher-throughput than image-based phenotypic assays. However, background noise interference in quality and usage of counter-screening assays to assess compound cytotoxicity and support conclusions should be considered. Conversely, HCS assays provide multi-parametric evaluation of both viral infection and cytotoxicity in same assay28,31. Therefore, in the present study we propose the development of a reproducible, phenotypic HCS for CHIKV, in order to trial drugs with antiviral activity.
Different approaches have been described to assess drugs in a high-throughput screening (HTS) format against CHIKV, including measurement of cell viability using a resazurin assay27, replicon-based assay using a Renilla luciferase reporter10,11,32,33, which targets only replication-process-interfering compounds, or a HCS assay using BHK cells9. In this study, we opted for the Huh-7 cell line as this has been used for HCS for antiviral discovery by our group and others for hepatitis C34, dengue13 and Zika12,35. Moreover, it is reported that Huh-7 cells are permissive to CHIKV infection. The CHIKV viral cycle usually happens in a short period of time, between 8 and 16 hours, following high cytopathic effect36. In this manner, we opted to use a relatively low MOI (0.05) to prevent high cell lysis (Figure 1A), and it can be expected that multiple infection cycles happen during the assay duration (48 h). Thus, all potential targets during the whole viral cycle can be exposed to the compounds. The developed assay also proved to be robust and reproducible.
IFN-2αA, bafilomicyn A1, chloroquine and mycophenolic acid had all been previously reported as active against CHIKV in vitro21,22,37, and their antiviral activity was confirmed under the conditions used in this study. However, bafilomycin A1 and chloroquine were cytotoxic, resulting in low SIs (<5). Bafilomicyin A1, an inhibitor of mammalian vacuolar-type H(+)-ATPase, prevents the acidification of the endosomal compartment, where the low pH allows the fusion of viral capsid followed by the entrance in the cytoplasm, thus preventing a crucial early step of the CHIKV virus cycle38. The same inhibition mechanism was observed in vitro during for the infection of sindbis virus, a prototype alphavirus39. Previous studies have reported the cytotoxicity of bafilomicyin A1 in HEK123 cells21, as was also observed here in Huh-7 (Figure 3). The activity of chloroquine, an antimalarial compound, was extensively investigated against CHIKV, although studies in vivo with infected mice showed inefficient activity22,40. In addition, clinical trials comparing double-blinded placebo groups and patients with CHIKV infection group did not present convincing data regarding chloroquine treatment efficacy41. Studies in CHIKV-infected Vero cells suggested that chloroquine exerts antiviral activity by preventing CHIKV internalization. The chloroquine EC50 values observed in this study (21 μM) in Huh-7-infected cells are in accordance with values previously reported for Vero cells (17 μM)22. However, the cytotoxicity of chloroquine seems to vary depending on the cell type or assay conditions, as chloroquine showed greater cytotoxicity in Huh-7 cells (with values of CC50 of 56 µM) than Vero cells (CC50 >100 µM)22. Mycophenolic acid inhibits inosine monophosphate dehydrogenase (IMPDH), an essential enzyme in de novo biosynthesis of guanine, and has been reported to have antiviral activity for both single strand RNA negative and positive viruses, for instance, against influenza virus42, dengue virus43, and the alphavirus VEEV44. CHIKV activity was previously reported to have EC50 values of 0.1 µM in Vero cells37, and here was observed in Huh-7 EC50 of 0.8 µM, confirming values in similar levels (Figure 3) albeit with lower selectivity. Nevertheless, a human cell model, such as the Huh-7 cell line, should be preferred to screen antiviral candidates to promote a more representative values of activity and cytotoxicity, which may diverge when compared to non-human cells lines45.
Sofosbuvir, 5-fluorouracil, daclatasvir and ledispavir are all FDA-approved drugs with reported activity against flaviviruses. The nucleoside analog 5-flurouracil is used to treat neoplastic disease46, and we have recently shown its antiviral activity in vitro against ZIKV infection12. However, 5-flurouracil presented no activity against CHIKV in our assay, suggesting selectivity for flaviviruses. Comparable results were obtained for ledispavir, which did not show inhibition against CHIKV, even at the highest concentration tested. Ledispavir, daclatasvir and sofosbuvir are direct-acting antiviral agents and have been successfully used to treat HCV-infected subjects. Those compounds target NS5A and NS5B, two HCV non-structural proteins47. NS5A presents three domains, which are responsible for genome replication, virus assembly through production of infection virus particles, and regulation of viral genome replication, from direct interaction of NS5A domain II with NS5B. NS5B is an RNA-dependent RNA-polymerase (RdRp) that directs the RNA synthesis in the HCV replication cycle48. Ledispavir and daclatasvir are NS5A inhibitors, while sofosbuvir, an uridine nucleoside analog that targets NS5B that is usually administrated in combination with daclatasvir49. Besides HCV, recent studies have demonstrated that sofosbuvir can inhibit infection by other flavivirus in vitro and in mice25,26. Our results demonstrated sofosbuvir elicited a concentration-dependent inhibition of CHIKV infection (Figure 3 and Figure 4), suggesting that this drug might have a broader antiviral spectrum than previously known.
Differently from HCV, which the genome organization consisted in five non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A50), CHIKV possess four non-structural protein (nsP1, nsP2, nsP3 and nsP4), being the RdRp domain localized at nsP4. Alignment sequence of RdRp has demonstrated highly conserved regions between CHIKV and other flaviviruses. More specifically, the motif B region, which is a functional domain of viral RdRp coding region, and the R1 motif, which has a role in nucleoside triphosphate binding during viral RNA synthesis, are highly conserved. Besides, CHIKV RdRp forms similar structures to the RdRp of other RNA viruses51. The search for direct-target compounds against CHIKV have focused on nsP2, due to its multifunctioning domains, which acts as helicases to form RNA secondary structures, as triphosphates responsible for RNA capping enzyme and removing terminal phosphate from new RNA template, and as proteases responsible for processing non-structural polyproteins52. In addition, its well-known structure makes nsP2 a suitable target for drug design53. However, few studies have focused on the search for compounds that target RdRp for CHIKV54,55. A compound that targets RdRp would be attractive, as RdRp acts on a viral process, is essential for replication of the viral genome and does not affect host cells55.
In conclusion, the phenotypic high content analysis established herein revealed that sofosbuvir is a promising candidate for use against CHIKV infection. Further studies should be performed in order to elucidate the exact mechanism related to CHIKV RdRp inhibition by sofosbuvir.
Dataset 1. All raw data from the present study. Raw data are separated according to the figure in which they are presented; a guide to the data is available as a .docx file. DOI: https://doi.org/10.5256/f1000research.16498.d22190556.
This work has been supported by the Sao Paulo State Research Foundation - FAPESP (Process no. 2016/03780-5).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank Dr Paolo Zanotto, who provided the virology laboratory for performance of all experiments at Department of Microbiology, ICB-USP. We thank Denise Pilger for technical assistance, Dr. Amilcar Tanuri for supplying Huh-7 and Vero cells lines, Microbiológica Química e Farmacêutica for supplying sofosbuvir and daclatasvir, MedChemExpress (USA) for supplying Ledispavir and the World Reference Center for Emerging Viruses and Arboviruses for support to obtain the CHIKV 181/25 vaccine strain.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virology
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
Competing Interests: No competing interests were disclosed.
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
No
Are sufficient details provided to allow replication of the method development and its use by others?
Yes
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 31 Oct 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)