Keywords
bioanalytical methods, hypothalamic-pituitary-adrenal (HPA) axis, prenatal programming, steroids, ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS)
bioanalytical methods, hypothalamic-pituitary-adrenal (HPA) axis, prenatal programming, steroids, ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS)
Amniotic fluid, with its nutritive and protective functions, is essential for fetal well-being and development1. It is contained by the amniotic cavity enclosed by the amnion and chorion2. Amniotic fluid originally comes from maternal plasma and passes through the fetal membranes, being fueled by secretions from the fetal respiratory and gastrointestinal tract, from the oral cavity, and from fetal skin before keratinization occurs1,3–5. Furthermore, cells from the amnion layers secrete proteins into the amniotic fluid2. At the beginning of pregnancy, the biochemical composition of human amniotic fluid resembles fetal extracellular fluid, probably due to passage across the still unkeratinized fetal skin6. Given the interplay between amniotic fluid and fetal physiology, amniotic fluid has been used as an important source of potential biomarkers for fetal pathologies7.
Fetal endogenous steroids, including cortisol, cortisone, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), estradiol, estriol, and testosterone are involved in broad and essential physiological functions, including glucose- and energy-metabolism, development, timing of pregnancy, adaptation to the extra uterine environment, and immune processes [e.g. 8,9]. Dysfunction or dysregulation of underlying hormone systems (e.g. the hypothalamic-pituitary-adrenal axis) is seen in major endocrine diseases (e.g. Cushing’s syndrome, Addison’s disease)10,11 and non-endocrine disorders (including mental disorders and stress-related conditions)12–16. Further, information on prenatal steroid hormone concentrations is of relevance for elucidating fetal origins of health, disease, and variation in mental processes17–24. Hence, the assessment of these hormones is of clinical and scientific relevance for a range of different disciplines, including endocrinology, psychiatry, psychobiology, psychology, neonatology, pediatrics, gynecology, and internal medicine [e.g. 22].
Therefore, there is a strong need for powerful and easily applicable analytical procedures for research as well as diagnostic purposes, to assess fetal steroids in amniotic fluid, overcoming previous limitations of sensitivity and specificity. However, as yet, respective methods are scarce. Steroid hormones can be quantified by traditional immunoassays but with several limitations as a lack of sensitivity making the quantification of small amounts of steroids difficult or a lack of specificity due to antibody cross-reactivity resulting in results higher than true concentrations25. The presence of interfering substances such as autoantibodies, exogenous substances, hemolysis or lipaemia in patient samples can alter the measurable concentration of the analyte26. The matrix effect problems also affect immunoassays. Furthermore, a relatively large sample volume is required as immunoanalysis provides only single-analyte assays and this is particularly a problem for precious samples such as amniotic fluid. Mass-spectrometric techniques are an alternative to address immunoassays issues. It allows for highly sensitive and specific simultaneous assessment of multiple biomarkers, for example in the context of metabolic and proteomic profiling in amniotic fluid7,25. Recently, first studies reported on the successful application of different mass-spectrometric approaches, including gas-chromatography mass spectrometry (GC-MS) and liquid-chromatography tandem mass spectrometry (LC-MS/MS), for concomitant assessment of multiple selected steroids in amniotic fluid26–28.
The main objective of this study was to develop highly sensitive and specific analytical ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) methods for determination of cortisol, cortisone, DHEA, DHEAS, estradiol, estriol, and testosterone concentrations in human amniotic fluid, and provide proof-of-principle for the method’s applicability in amniotic fluid samples of pregnant women, collected within obstetric care.
282 pregnant women were recruited via Prenatal Medicine and Genetics, Duesseldorf, Germany, who were undergoing regular amniocenteses for diagnostic reasons with maternal age as the only risk factor. Samples from seven women were not included in the analyses because of problems with either the consent forms or the amniotic fluid samples. Sociodemographic and obstetric characteristics of the 275 women included in the analyses are presented in Table 1. Amniotic fluid samples were collected in the years 2010 to 2012, between week 14 and week 18 of gestation. Samples were collected, using GREINER BIO-ONE (Frickenhausen, GERMANY) tubes CRYO’S 124 and stored at –80°C for later analyses. Biochemical analyses were conducted in 2013 at the laboratory of the Institute of Legal Medicine at the University of Strasbourg, France.
| Normally distributed continuous variables: Mean (standard deviation) [unknown] | |
|---|---|
| Maternal age at time of amniotic fluid collection (years) | 37.70 (3.52) |
| Offspring’s weight at birth (g) | 3362.46 (507.84) |
| Offspring’s body length at birth (cm) | 51.45 (2.89) |
| Total length of gestation (weeks) | 38.91 (1.68) |
| Other continuous variables: Median (range) [unknown] | |
| Length of gestation at time of amniotic fluid collection (weeks) | 15 (14–18) |
| Discrete variables: N (%)1 | |
| Singleton/multiple pregnancy Singleton pregnancy Twin or higher order pregnancy Unknown | 83.5% 1.8% 14.7% |
| Offspring sex Male Female Unknown | 50.2% 45.8% 4.0% |
| Mode of delivery Spontaneous birth Forceps delivery/vacuum extraction Cesarean section Unknown | 48.8% 3.6% 41.2% 6.5% |
All participants provided written informed consent that their samples were used in research after genetic examinations. The study protocol was approved by the Ethical Committee of Faculty of Science and Mathematics, Heinrich-Heine-University, Duesseldorf, Germany.
Chemicals. Dichloromethane was purchased from Carlo Erba (Val de Reuil, France). HPLC-grade methanol (MeOH), isopropanol, diethyl ether, ammonium hydroxide (NH4OH) and formic acid (HCOOH) were purchased from VWR (Fontenay-sous-bois, France). HPLC-grade acetonitrile (ACN) was obtained from Merck (Darmstadt, Germany) and Oasis HLB (30mg/30µm) solid-phase microextraction plate was obtained from Waters (Milford, USA). Sodium bicarbonate (NaHCO3), dansyl-chloride, cortisol, cortisone, DHEA, testosterone, estradiol and estriol were obtained from Sigma (Saint-Quentin Fallavier, France) while deuterated cortisol (cortisol-d4, 9, 11, 12, 12 D) and DHEAS were obtained from Steraloids (Newport, USA).
Solution preparation. Cortisol, testosterone and estradiol solutions were prepared in MeOH at a final concentration of 2.5, 25 and 250 ng/mL. Cortisone, DHEAS, estriol solutions were prepared in MeOH at a final concentration of 2.5, 25, 250 and 2500 ng/mL. DHEA solutions were prepared in MeOH at a final concentration of 0.025, 0.25, and 2.5 and 25 µg/mL. Deuterated cortisol was prepared in MeOH at a final concentration of 250 ng/mL. These solutions were stable for at least 6 months at 4°C. Sorensen buffer was prepared by adding 38.8 mL KH2PO4 (9.07 g/L) to 6.12 mL Na2HPO4 (11.87 g/L); pH value was adjusted to pH 7.6. NaHCO3 buffer was prepared by adding 1 L of distilled water to 8,4 g of NaHCO3; pH value was adjusted to pH 10.5. Dansyl chloride solution was prepared by adding 20 mL of acetone to 20 mg of dansyl chloride.
Calibration standards and quality control. Distilled water was used for calibration standards. All molecules of interest were thus not detectable in the blank samples. Calibration standards were prepared at concentrations ranging from limit-of-quantification (LOQ) to high concentration for each analyte. Intra- and inter-assay precision were determined by enriching 250 µL of distilled water with the analytes of interest at three concentrations (low, medium and high) for each hormone.
Sample preparation for cortisol, cortisone, DHEA, DHEAS and testosterone determination. 0.25 mL of amniotic fluid were mixed in 0.75 mL Sorensen buffer (pH 7.6) in the presence of 20 ng/mL cortisol-d4 as internal standard. For further purification, SPME Oasis® HLB extraction plates were used. Activation was operated with 0.2 mL MeOH, followed by 0.2 mL deionized water. The incubation medium was centrifuged and the supernatant was removed and deposited on the activated plate, then rinsed with 0.2 mL deionized water/MeOH (95:5, v/v). The plate was allowed to dry for 5 minutes at room temperature. Analytes were eluted with 35 µL ACN/isopropanol (40:60, v/v) with 2% of concentrated NH4OH, followed by 35 µL deionized water. Ten µL of this extract were directly injected into the UPLC-MS/MS system.
Sample preparation for estradiol and estriol determination. 3 mL of diethylether was added to 0.25 mL of amniotic fluid in the presence of 20 ng/mL cortisol-d4 as internal standard. Samples were agitated 15 minutes. Samples were centrifuged 15 minutes at 3000g and the organic phase was transferred into a new glass tube. The supernatant was evaporated at 40°C under a constant stream of nitrogen until the samples were completely dried. Finally, 50 µL of NaHCO3 buffer (pH 10.5) and 50µL of dansyl-chloride solution were added and the tube was vortexed for 30 second. The mix was heated 3 minutes at 60°C. Ten µL of this extract were directly injected into the UPLS-MS/MS system.
Chromatographic and mass spectrometric conditions. A Waters (Milford, USA) Acquity UPLC system with a column heater, autosampler, and a 10µL injection loop was used. Analytes were separated at 30°C on a Waters Acquity UPLC BEH C18 column (1.7 µm, 100 x, 2.1-mm). Separation was achieved by gradient elution with 0.1% HCOOH (pH 2.6) and ACN at a flow rate of 0.4 mL/min (0 to 6 min, 90% HCOOH and 10% ACN; 6 to 9 min, 40% HCOOH and 60% ACN; 9 to 11 min, 90% HCOOH and 10% ACN). The total run time was 11 minutes, including periods required for injection and equilibrating the column before the next injection.
Detection was carried out by a Quattro Premier XE tandem mass spectrometer (MS/MS) (Waters Micromass, Manchester, UK). This mass spectrometer was equipped with an electrospray ionization probe and operated switching between positive and negative ionization mode. The voltage of the capillary was 3.5 kV in positive mode. The ion-source temperature was 120°C and the desolvation gas was heated to 400°C at a flow rate of 800L/h. Quantitative results were obtained in MRM (Multiple Reactions Monitoring) mode after determination of the transition for each glucocorticoid: cortisone m/z 361.1>163.1, cortisol m/z 363.2>121.1, DHEA m/z 289.2>253.2, DHEAS m/z 367.1>96.9 (ES-), testosterone 289.2>97.1, estriol 522.3>170.9 and estradiol 506.3>170.9, against cortisol-d4 m/z 367.2 > 121.1. Qualifier ions were monitored, being m/z 361.1>121.1 for cortisone; m/z 363.2>309.1 and m/z 363.2>327.1 for cortisol; m/z 289.1>213.1 for DHEA; m/z 289.2>109.1 for testosterone, m/z 522.3>155.0 for estriol and m/z 506.3>155.9 for estradiol. Masslynx®4.1 software (Waters) was used for data acquisition.
The following parameters were tested to validate the method: limit-of-detection (LOD), LOQ, linearity, recovery, intra- and inter-assay precision.
The LOD and LOQ were determined by analysis of replicate blank samples (n=6) spiked with hormones at various concentrations. The LOD and LOQ were estimated as giving a signal-to-noise ratio greater than 3 and 10 respectively, for each of the quantitative ions transitions monitored.
Linearity was tested by the preparation of calibration curves ranging from the LOQ to 20 ng/mL for cortisol, to 75 ng/mL for cortisone, to 500 ng/mL for DHEA, to 50 ng/mL for DHEAS, to 10 ng/mL for testosterone, to 100 ng/mL for estriol and to 25 ng/mL for estradiol. Three curves were developed for 9 concentration levels of each hormone. Linearity of the method was expressed by the correlation coefficient (r2).
Analyte recovery of the extraction procedure was determined by analyzing replicate blank samples (n=3) spiked with hormone (LOQ, medium and high concentration) against replicate blank extracts (n=3) spiked at the same levels after extraction.
Precision was evaluated using three solutions with all hormones (LOQ, medium and high concentration) and expressed as coefficient of variation (CV). The intra-assay precision was assessed by determining these samples on one day (n=10 for each sample), while the inter-assay precision was assessed over 8 days (n=8 for each sample).
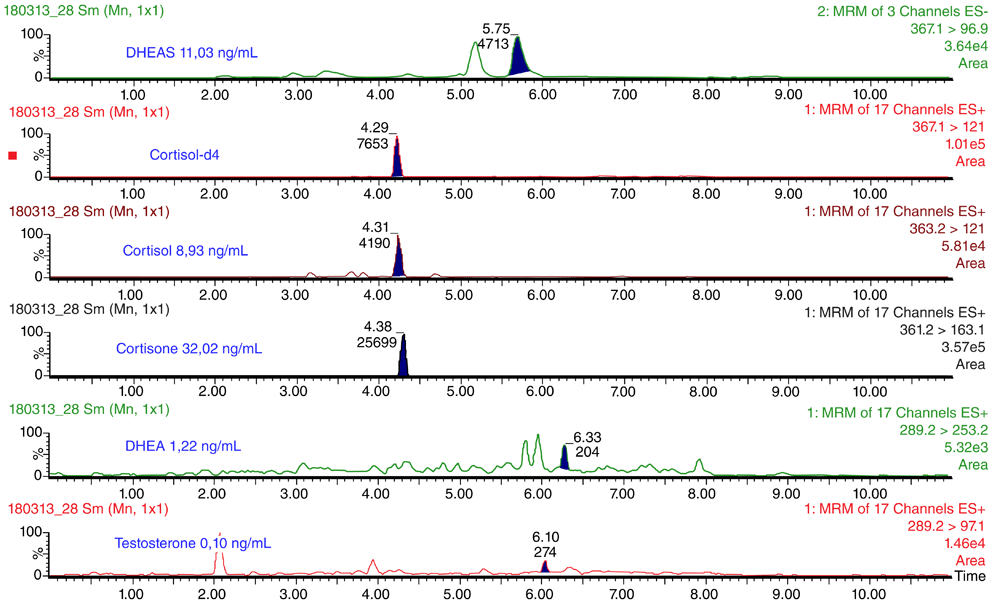
Under the UPLC conditions described above, cortisol, cortisone, DHEA, DHEAS and testosterone were sufficiently separated chromatographically (Figure 1). The mean (± standard deviation) retention times were 4.35 ± 0.05 min for cortisol, 4.40 ± 0.04 min for cortisone, 6.35 ± 0.04 min for DHEA, 5.75 ± 0.04 min for DHEAS 6.13 ± 0.04 min for testosterone and 4.38 ± 0.08 min for cortisol-d4. Estradiol and estriol were separated chromatographically under UPLC conditions (Figure 2). The retention times were 8.00 ± 0.04 min for estradiol and 7.16 ± 0.04 min for estriol.

Cortisol, cortisone, DHEA, DHEAS, testosterone assessment
LOD, LOQ and linearity
The LOD and LOQ, as well as the CVs are presented in Table 2, for both cortisol, cortisone, DHEA, DHEAS, testosterone.
| Cortisol | Cortisone | DHEA | DHEAS | Testosterone | |
|---|---|---|---|---|---|
| LOD (ng/mL, n=6) | 0.02 | 0.005 | 0.75 | 0.02 | 0.02 |
| CV (%) | 18.98 | 19.12 | 19.45 | 19.34 | 19.20 |
| LOQ (ng/mL, n=6) | 0.05 | 0.01 | 1.0 | 0.05 | 0.05 |
| CV (%) | 18.43 | 18.23 | 19.31 | 19.67 | 19.54 |
The calibration curve obtained showed good linear responses with a r2 of 0.9974, 0.9907, 0.9956, 0.9856 and 0.9940 for cortisol, cortisone, DHEA, DHEAS and testosterone respectively from the range of 0.05 to 20 ng/mL for cortisol, 0.01 to 75 ng/mL for cortisone, 1 to 500 ng/mL for DHEA, 0.05 to 50 ng/mL for DHEAS and 0.05 to 10 ng/mL for testosterone.
Extraction recovery
Table 3 shows the recovery tested for each analyte at three different concentrations (LOQ, medium and high concentrations). The extraction recoveries were > 71%, > 86%, > 73%, > 80% and > 52% for cortisol, cortisone, DHEA, DHEAS and testosterone respectively.
Precision
Intra- (n=10) and inter-assay (n=8) precision were evaluated for each hormone at 3 concentrations (LOQ, medium, and high). The intra-assay (n=10) precision values were less than 20% for all hormones at LOQ and less than 15% for all hormones at medium and high concentration. Good inter-assay (n=8) values (less than 20% for both analytes at LOQ and less than 15% at other medium and high concentrations) were obtained, as outlined in Table 4.
Estradiol and estriol assessment
LOD, LOQ and linearity
The LOD and LOQ, as well as the CVs are presented in Table 5, for both estradiol and estriol.
| Estradiol | Estriol | |
|---|---|---|
| LOD ng/mL, n=6) | 0.02 | 0.005 |
| CV (%) | 19.32 | 19.56 |
| LOQ ng/mL, n=6) | 0.05 | 0.01 |
| CV (%) | 18.87 | 17.39 |
The calibration curves showed good linear responses with a r2 of 0.9967 and 0.9979 for estradiol and estriol respectively, within the range of 0.01 to 25 ng/mL for estradiol and 0.05 to 100 ng/mL for estriol.
Extraction recovery
Table 6 shows the recovery tested for each analyte at three different concentrations (LOQ, medium and high). The extraction recoveries were > 77% and > 92% for estradiol and estriol respectively.
| Recovery (n=6) | |||
|---|---|---|---|
| Compound | ng/mL | % | CV (%) |
| Estradiol | 0.05 | 77.83 | 18.07 |
| 0.1 | 88.83 | 14.60 | |
| 0.5 | 92.44 | 13.87 | |
| Estriol | 0.05 | 94.98 | 18.00 |
| 0.5 | 92.51 | 12.98 | |
| 5 | 98.28 | 12.28 | |
Precision
Intra- (n=10) and inter-assay (n=8) precision were evaluated for estradiol and estriol at 3 concentrations (LOQ, medium and high). The intra-assay (n=10) values were less than 16% for both estradiol and estriol and good inter-assay (n=8) values (less than 17% for both analytes) were obtained, as demonstrated in Table 7.
| Intra-assay (n=10) | Inter-assay (n=6) | ||
|---|---|---|---|
| Compound | ng/mL | CV (%) | CV (%) |
| Estradiol | 0.05 | 14.44 | 16.38 |
| 0.1 | 12.53 | 12.09 | |
| 0.5 | 12.15 | 11.62 | |
| Estriol | 0.05 | 15.12 | 14.93 |
| 0.5 | 12.27 | 14.82 | |
| 5 | 12.32 | 13.01 |
Application on amniotic fluid samples. UPLC-MS/MS analyses were performed on 275 amniotic fluid samples (see Table 8, Figure 3 and Figure 4). Cortisol, cortisone, DHEAS, estradiol and estriol concentrations were quantified in all samples. DHEA was quantified in 11 of the 275 samples analyzed. Testosterone was quantified in 119 of the 275 samples analyzed (samples from pregnancies with male offspring – all twin or higher order pregnancies resulted in male offspring only: quantified in 116 out of 138 samples analyzed; samples from pregnancies with female offspring: quantified in 3 out of 126 samples analyzed). For DHEA, in 35 of the analyzed samples, concentrations were below the LOQ of 1 ng/mL but above the limit-of-detection of 0.75 ng/mL. For testosterone, in 39 of the analyzed samples, concentrations were below the LOQ of 0.05 ng/mL but above the limit-of-detection of 0.02 ng/mL.
| Minimum | Maximum | Median | |
|---|---|---|---|
| Compound | ng/mL | ng/mL | ng/mL |
| Cortisol | 0.90 | 10.03 | 3.24 |
| Cortisone | 3.32 | 52.58 | 11.63 |
| DHEA1 | 1.05 | 1.86 | 1.22 |
| DHEAS | 0.85 | 22.57 | 3.93 |
| Testosterone2 | 0.05 | 0.34 | 0.09 |
| Estradiol | 0.05 | 0.76 | 0.12 |
| Estriol | 0.10 | 2.24 | 0.50 |
We developed an analytical method to simultaneously measure cortisol, cortisone, DHEAS, and testosterone, and an analytical method to simultaneously measure estradiol and estriol in human amniotic fluid, using an LC-MS/MS assay, with good linearity, sensitivity, specificity and accuracy without interferences between the molecules and a low LOQ. Five out of the seven hormones were quantifiable in all amniotic fluid samples, while DHEA was quantifiable only in a few samples and testosterone was primarily quantifiable in samples from women carrying a male fetus.
The results add to previous findings that indicated that different mass-spectrometric approaches, including gas-chromatography mass spectrometry (GC-MS) and LC-MS/MS, can be applied for assessment of steroids in amniotic fluid27,28.
Comparisons of our results on steroid concentrations in human amniotic fluid with data from previous studies are hampered by substantial heterogeneity across previous reports. For example, even when restricting comparisons to findings also based on LC-MS/MS, cortisol concentrations in human amniotic fluid have previously been reported as higher28 as well as substantially lower29 than the here reported values. Reasons for such heterogeneity may include – besides the analytical procedures themselves – differences across studies with regard to i) indication for amniotic fluid collection, ii) gestational age at collection, iii) other sociodemographic or obstetric characteristics related to the pregnancy, iv) procedures applied for amniotic fluid collection and storage, and v) the interval between collection and biochemical analyses of amniotic fluid [cf. 1,29–31]. Overall, the here reported concentrations are compatible in magnitude with previous findings on cortisol, cortisone, DHEA, DHEA-S, and testosterone concentrations in human amniotic fluid, assessed via mass spectrometric approaches27,28, and with previous findings on estradiol and estriol concentrations in human amniotic fluid, assessed via immunoassay-based approaches32,33 (we are not aware of any respective information from previous studies using mass spectrometric approaches).
Our study has several strengths. We developed analytical methods to quantify a range of steroid hormones in human amniotic fluid with low minimal amount of sample and a total analytical run time of 11 minutes, allowing simultaneous assessment of several hormones. Using state-of-the-art approaches, we confirmed high sensitivity and specificity, good accuracy and reproducibility of the analyses. Notably, the UPLC-MS/MS methods show no interference in contrast to many immunoassays. Last but not lest, by providing proof-of-principle for using the methods to quantify hormone concentrations in human amniotic fluid samples, we open the way for their further development for a wide range of potential applications in scientific and clinical settings.
Our study has several limitations. First, for one out of the seven analytes, we were unable to quantify the steroids in all samples and for another one out of the seven analytes, we were unable only in samples from females to quantify the steroids, most likely because concentrations in the respective samples were below our LOQ or even LOD. For DHEA, the analytical method was not sufficiently sensitive to detect a concentration under 0.75 ng/ml. Although recoveries were greater than 80%, detection limits of the UPLC-MS/MS system have been reached for this molecule. The analytical study of steroids in amniotic fluid of midgestation made by Fahlbusch et al. showed DHEA concentrations of 0.64 ± 0.48 ng/mL for male group of fetuses and 0.56 ± 0.36 ng/mL for female group of fetuses28. According to these results, LOQ should be at least 0.1 ng/mL to detect DHEA in 275 samples analyzed. Similarly, the lack of analytical sensitivity also affects testosterone results. According to Fahlbusch et al., testosterone concentrations in amniotic fluid were 0.30 ± 0.15 ng/mL for male group of fetuses and 0.02 ± 0.02 ng/mL for female fetuses30. Kushnir et al. determined serum pediatric references ranges below 0.37 ng/mL for males aged 6 to 24 months and below 0.09 ng/mL for females of similar age34. In the same, Soldin et al. showed testosterone concentrations in serum range from 0.04 to 0.31 ng/mL for males (0–6 years) and range from 0.02 to 0.1 ng/mL for females (0–5 years)35. In view of these studies, the LOQ of our method was not sensitive enough to detect a low concentration of testosterone in amniotic fluid particularly for female fetuses. However, identifying a concentration as being below LOQ may also be of diagnostic value. Second, timing of amniotic fluid collection was not evenly distributed across pregnancy, so we cannot draw conclusions regarding changes in amniotic fluid steroid concentrations throughout gestation. Third, amniotic fluid samples were not immediately assessed after collection, so we cannot exclude degradation of analytes between sample collection and analysis, even though storage conditions would have made substantial degradation rather unlikely. Finally, as amniotic fluid is not routinely collected during every pregnancy, we cannot assume that our sample of study participants is representative with regard to the population of pregnant women. However, we drew our participants from women, undergoing amniotic fluid collection during obstetric care just for the reason that the pregnant women were above 30 years of age. Hence we expect our sample of study participants being largely comparable to a typical sample of women undergoing amniotic fluid testing.
Future studies should further address the role of storing conditions and time interval between collection and analyses. Moreover, future studies should aim at further increasing the sensitivity of the methods. Finally, further studies should test the functional significance of the methods and provide data on their validity and applicability in clinical and research settings.
The methods may provide new opportunities for future applications in a range of fields, such as endocrinology and beyond, providing a tool to determine steroid concentrations in pathological conditions characterized by disturbances of steroid hormones and related enzymatic activities.
We here demonstrated that cortisol, cortisone, DHEAS, estradiol, estriol, and testosterone concentrations are easily detectable and quantifiable in human amniotic fluid, using state-of-the-art and reproducible LC-MS/MS techniques with good linearity, sensitivity, specificity, and accuracy without interferences for the molecules and with a low LOQ. The methods may provide an appropriate approach for a wide range of clinical and research applications, including the field of endocrinology and others.
Individual (non-aggregated) data cannot be made publicly available, due to ethical restrictions. In order to access this data, data must be requested from the corresponding author. Data requestors will have to provide: i) written description and legally binding confirmation that their data use is within the scope of the study, as outlined in the ethical board request and written informed consent (respective information will be provided by the corresponding author on request); ii) detailed written description and legally binding confirmation of their actions to be taken to protect the data (e.g., with regard to transfer, storage, back-up, destruction, misuse, and use by other parties), as legally required and to current national and international standards (data protection concept); and iii) legally binding and written confirmation and description that their use of these data is in line with all applicable national and international laws (e.g., the General Data Protection Regulation of the EU). In case of requests of hormone data only, less restrictive regulations may apply.
GM received funding from the Swiss National Science Foundation (SNSF) under project no. 100014_135328, funding from the Stanley Thomas Johnson Stiftung & Gottfried und Julia Bangerter-Rhyner-Stiftung under project no. PC_28/17, funding from the Forschungsfonds of the International Psychoanalytic University Berlin, and funding from the Swiss Cancer League KLS-4304-08-2017. MT receives funding from the SNSF under project no. PZ00P1_137023, and GM and MT receive funding from the Korea Research Foundation within the Global Research Network Program under project no. 2013S1A2A2035364.
The funders had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
No
Are sufficient details provided to allow replication of the method development and its use by others?
No
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: LC-MS/MS method development
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 02 Nov 18 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)