Introduction
Cardiovascular diseases represent a prevalent medical challenge and are a leading cause of morbidity and mortality in developed societies. Although multiple approaches have been developed in the last two decades to repair or replace damaged myocardium, progress to date has been modest and cardiomyocyte replacement in adult hearts remains elusive. While understanding the loss of cardiac muscle and its eventual replacement is an important goal for cardiac research, there are additional cellular targets that hold promise. These include abrogating scar tissue formation following heart injury or promoting robust revascularization to the injured heart tissue. Here, we present an overview of the different cell types that participate in mammalian heart development as well as how the microenvironment participates in cardiac remodeling. Typically, tissue repair is driven by tissue-resident progenitors. Although significant debate exists regarding the identity and functional potential of adult cardiac progenitor cells (CPCs), there is an emerging consensus that the adult epicardium contains cells with progenitor potential that can participate in cardiac remodeling after injury. Whereas the heart undergoes profound changes during embryonic, fetal, and early postnatal growth, the epicardium maintains many characteristics found during heart development. Consequently, the adult epicardium is an important cellular compartment that may provide solutions to ameliorating heart repair. We focus particularly on rodent models that have allowed the identification of progenitor populations that give rise to the heart as well as for the study of specific cell types in response to injury.
Cardiac progenitor populations during development
The mature heart consists of an inner layer of endocardial cells (endocardium) and an outer layer of epicardial cells (epicardium) that surround the myocardium. The myocardium is composed of cardiomyocytes as well as cells of the conductive system, smooth muscle, and endothelium and stromal cells/fibroblasts and valvular interstitial cells1,2. Three sets of multipotent precursors have been identified that give rise to cardiac cells: cardiogenic mesoderm cells3,4, cardiac neural crest cells (cNCCs)5, and proepicardial organ (PEO) cells6,7 (Figure 1A). Cardiogenic mesoderm cells give rise to cardiomyocytes by progressive lineage restriction8 that segregate during the onset of gastrulation9. During murine development, a first lineage appears at embryonic day 7.0 (E7.0), forms the cardiac crescent (first heart field, or FHF)10,11, and is the primary source of the left ventricular cardiomyocytes and primitive atria9,12. While the cardiac tube begins to contract and pump blood throughout the embryo13,14, a second lineage appears at E8.0 that constitutes the second heart field (SHF) and gives rise to the right ventricle, both atria, and the outflow tract (OFT)9,12,15–17. Unlike the FHF, the SHF consists of multipotent cells that give rise to cardiomyocytes as well as endothelial cells16,18–22 and the smooth muscle of the OFT region20,23,24. The multipotent capacity of the SHF has been assessed by using embryonic stem cells20 or in vivo analyses at the population level16,21,25; thus, the precise nature and clonal behavior of the SHF progenitors warrant further investigation. At the looping heart tube stage, when both FHF and SHF progenitors are in place, myocardium trabeculation occurs, leading to an increase of the endocardial and myocardial surface area that promotes oxygenation of the tissue26,27. The base of the trabeculae contains a population of proliferative cells—referred to as the compact layer—that are essential to ventricular wall expansion9,28–30. It has been proposed that the epicardium secretes mitogenic signals that drive cardiomyocyte proliferation in the compact zone31–33 (Figures 1A, 1B).
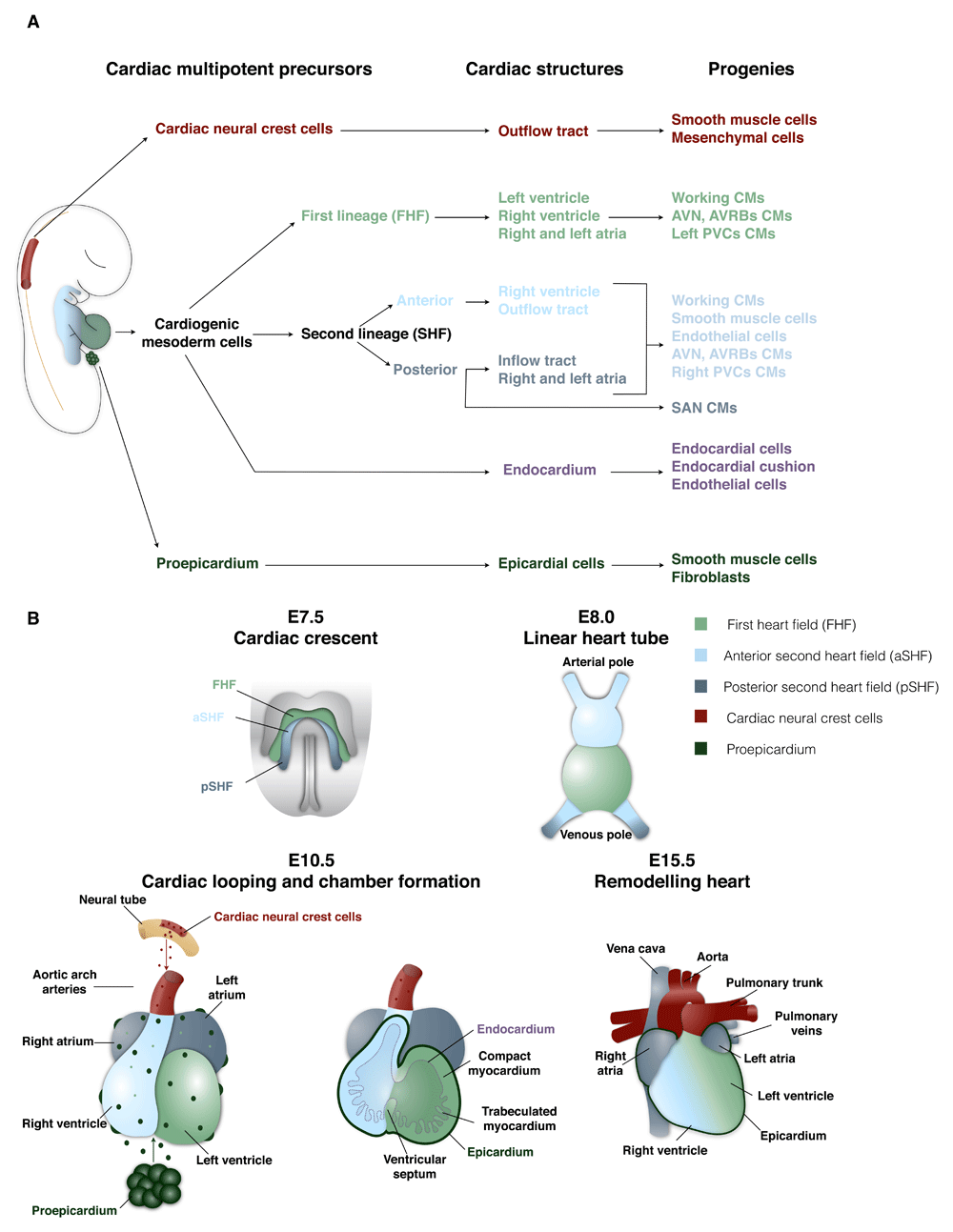
Figure 1. Patterning of mouse heart development.
(A) Hierarchical relationship of the different cardiac developmental progenitors and their progeny. (B) Main developmental steps of heart morphogenesis. A color code was assigned and followed to define each cardiac progenitor population and their progeny. AVN, atrioventricular node; AVRB, atrioventricular ring bundle; CM, cardiomyocyte; E, embryonic day; FHF, first heart field; PVC, peripheral ventricular conduction system; SAN, sinoatrial node; SHF, second heart field.
Another cellular compartment derived from cardiogenic mesoderm SHF consists of specialized endothelial cells (endocardium) that cover the inner surface of the cardiac tube and participate in different heart morphogenesis processes, which include trabeculation and a part of the coronary tree formation16,34–36. These two developmental events are intimately related, as the endocardium is essential to promote trabeculation of the ventricles27,37,38 and participates in the exchange of gas and nutrients with the blood circulating in the ventricular chambers27,34,39,40. During septation, ventricular trabeculae initiate compaction, increasing the thickness of the ventricular wall27,41,42. Endocardial cells surrounding the trabeculae become entrapped in the compacted myocardium and adopt a capillary-like morphology43–45. The newly formed endocardial-derived capillaries subsequently connect with the epicardial-derived vascular network. In addition, the endocardium is an important source of mesenchymal cells for endocardial cushion development46 and subsequent valvulogenesis and membranous septation47–49. During the septation, endocardial cells undergo an endothelial-to-mesenchymal transition (EndoMT) and colonize the subjacent cardiac jelly at the level of the auriculo-ventricular canal at E9.5 and the OFT at later stages50–52 (Figures 1A, 1B).
Cardiac neural crest derived from the dorsal neural tube at E9.5 migrates through the pharyngeal arches and gives rise to smooth muscle and mesenchymal cells that participate in the septation of the OFT trunk5,53 (Figure 1A, B).
The PEO is an extra-cardiac transient cauliflower-like structure that appears between E8.5 and E10.554 adjacent to the venous pole of the heart tube (splanchnic mesoderm-derived)6,55. PEO cells attach to the myocardium surface and spread out to constitute to the outer layer of the looping heart tube (that is, epicardium)56. A subset of epicardial cells undergoes epithelial-to-mesenchymal transition (EMT) in the subepicardial region referred to as mesenchymal epicardium-derived cells (EPDCs). A small fraction of EPDCs invades the myocardium to give rise to stromal cells/interstitial fibroblasts and coronary vasculature. The contribution of the PEO to cardiomyocytes and endothelial cells has been proposed but remains less clear31,57–61. An infrequent contribution to cardiomyocytes by the epicardium has been reported to be restricted to specific cardiac zones (inter-ventricular septum and parts of the atrial myocardium)59,60,62. In addition, it has been shown recently that nascent coronary vasculature forms immediately subjacent to the epicardium (subepicardial zone) and contributes to a large proportion of the coronary arteries, veins, and capillaries in the myocardial compact layer61,63. Following the formation of the initial vascular plexus, the coronary vessels connect to the aorta64. Irrespective of the origin, the coronary vasculature is essential for the subsequent heart morphogenesis and embryonic viability such that deficient coronary development impairs myocardium compaction and leads to embryonic lethality28,65 (Figure 1A, B). The cardiac conduction system is composed of specialized cardiomyocytes that assemble into a complex and heterogeneous structure to make up a central conduction system located in the atria (sinoatrial node [SAN], atrioventricular ring bundles, and the atrioventricular node) and a peripheral conduction system in the ventricles (left and right branches of His bundle, LBB and RBB, and left and right peripheral conductive system or the Purkinje fibers)66. SAN cardiomyocytes derive from the posterior SHF; however, lineage analyses revealed an early lineage segregation from the atrial working cardiomyocyte progenitors66,67. Retrospective clonal analyses showed that the nlacz gene transmitted cells are organized into clusters of clonally related cardiomyocytes, which are composed of a mixture of both working and specialized conductive cardiomyocytes68. These nlacz clusters were found in all conductive system compartments, except in the SAN, indicating a common origin between the specialized conductive cardiomyocytes and their neighbor working cardiomyocytes66,68,69. Of note, this retrospective clonal analysis does not exclude the contribution of other progenitor populations to the conductive system69. Additionally, other cardiovascular progenitors—that is, PEO (EPDCs)70 and cNCCs71—have been suggested to contribute to the development of the conductive system; however, this participation remains controversial66,69,72 (Figures 1A, 1B).
Our understanding of heart development is that specific progenitors arise at discrete developmental stages originating from different embryonic structures to form the heart. Thus, although the development of a tissue and an understanding of the progenitors involved can shed light on whether CPCs exist in the adult tissue, the heart poses a considerably more complex situation. Indeed, the search for adult CPCs has remained difficult and at times contentious regarding their identity as well as their cell fate potentials. While many tissues are capable of robust regeneration following injury, the mammalian heart shows a limited capacity to repair, suggesting that, if CPCs exist, their capacities are extremely limited. One possible reason for this limited capacity is that the CPC microenvironment of the adult is not permissive and thus the microenvironment of the developing heart warrants further study.
The developmental cardiac progenitor microenvironment
Placental pregnant females are commonly exposed to normal levels of oxygen (normoxia, 21% oxygen), and in turn the placenta regulates the level of oxygen available during embryonic and fetal development (Box 1 and Figure 2). The microenvironment of the developing heart is characterized by low levels of oxygen (physiological hypoxia) (Box 2)73 that promotes glycolytic metabolism74,75. Oxygen tension is an important signal driving tissue development and maturation76–79. Several studies have shown that physiological hypoxia regulates multiple cellular processes, including stem cell maintenance, proliferation, and differentiation, particularly in the context of angiogenesis and formation of placenta, heart, cartilage, and bone78,80–83. In addition, the developing cardiovascular system is unable to homogeneously deliver nutrients and oxygen throughout the embryonic/fetal heart61,63,76,77,83. Until mid-gestation, the coronary vasculature is absent and there is a gradient of oxygenation established from the endocardium (more oxygenated and in direct contact with the blood from the chambers) to the epicardium (less oxygenated)61,63,83. The myocardial compact layer (epicardial side) is composed of immature cardiomyocytes undergoing higher levels of proliferation under lower oxygen tension as compared with cardiomyocytes in the trabeculae (endocardial side)28,30,83. Ultimately, the coronary vasculature is established to meet the increasing demands of the myocardial wall expansion44,61. It has been demonstrated that hypoxia in combination with vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) mediates the formation of the primary capillary plexus (angioblasts) as well as subsequent vasculogenesis (formation of new vessels) and angiogenesis (budding and sprouting from pre-existing vessels), leading to the establishment of a functional coronary artery tree84–87 (Figure 2).
Box 1. Placental regulation of fetal oxygen tension
A complex coordination exists between the fetal cardiovascular system and the placenta to ensure stable oxygen levels73,88–90. Low maternal blood oxygen levels, cardiovascular failure, or impairment of placenta formation and function can induce pathological fetal hypoxia89–92. In pathological hypoxia, the fetus responds by favoring circulation to vital organs such as the brain and heart. In addition, a concomitant activation-specific stress response (for example, Hif-dependent and vascular endothelial growth factor pathways)80,81,83 occurs. Chronic fetal pathological hypoxia induces heart septation defects93, myocardial wall thinning, chamber dilation, and epicardium detachment79 as well as decrease of cardiomyocyte proliferation and increase of apoptosis94. Of note, in utero stress is also driven by other environmental stressors, including malnutrition that culminates with cardiac malformations and increased predisposition to adult chronic diseases95–98.
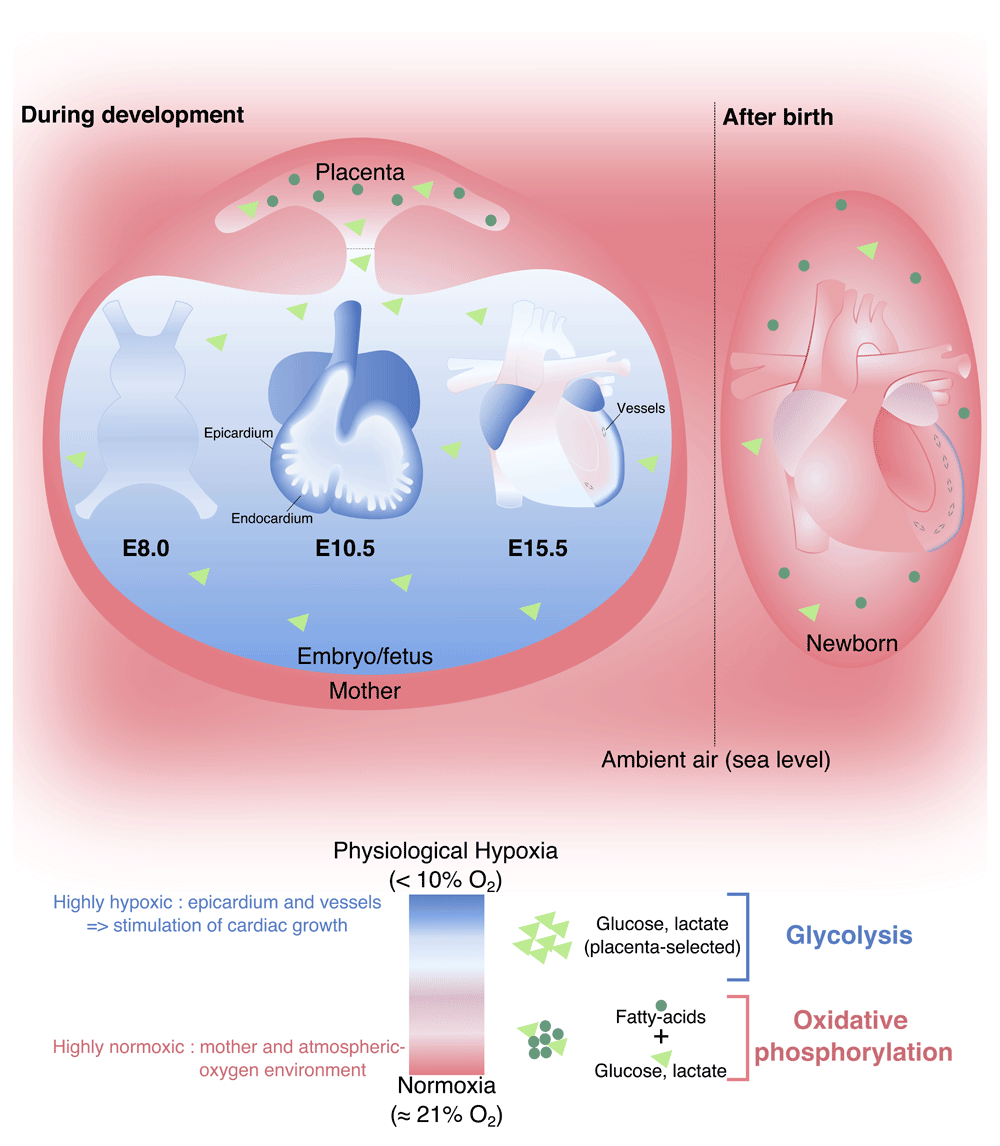
Figure 2. Placenta-selected hypoxic and glycolytic microenvironment and its impact on the heart development and metabolic switch after birth.
A color code and symbols were assigned and followed to define the microenvironment condition (depicted in the legend). E, embryonic day.
Box 2. Oxygen balance: normoxic and hypoxic environment
The ability to maintain oxygen homeostasis is essential for many biological processes, including cell survival and overall development and tissue maintenance. Specialized cells (for example, smooth muscle cells and carotid and neuroepithelial bodies) mobilize proteins and regulate gene expression in order to adapt to hyperoxia or hypoxia100–102. The environmental oxygen concentration at sea level is 21%103, whereas placental developmental occurs under lower oxygen conditions (2–8%)104. This physiological hypoxia is important for placental and fetal morphogenesis and growth73. During development, fetal arterial blood oxygen tension ranges between 22 and 32 mmHg but in the adult systemic tissues is about 80 to 100 mmHg93. Fetal arterial blood persistently contains less oxygen, suggesting that fetal development occurs in a state of relative hypoxia (that is, physiological hypoxia) in comparison with the adult73.
The murine heart is the first organ to function during development (E8.5)13,14, and the continuous myocardial contraction requires energy (adenosine triphosphate [ATP]) availability. About 80% of all energy produced by cardiac muscle is consumed by the mechanical activity of the heart, whereas heart morphogenesis relies on the remaining energy75. The developing heart relies primarily on carbohydrates (that is, glucose and lactate) as a source of ATP74,75. The placenta regulates the availability of metabolic substrates that are delivered to the embryonic/fetal circulation, whereas the fatty acids are blocked99. Glucose is transported to the cytosol of cardiomyocytes and used for glycolysis, glycogen synthesis, or pentosephosphate shunt (metabolic pathway parallel to glycolysis). Glycogen synthesis and storage in fetal cardiomyocytes have been shown to be important sources of phosphorylated glucose, which protects cardiomyocytes from hypoxia. During fetal development, cardiomyocytes have low mitochondrial content75,99. In summary, embryonic and fetal cardiac development occurs in a physiological hypoxic state, which is essential for the ability of cardiac progenitors to proliferate, self-renew, and differentiate (for example, immature cardiomyocyte hyperplasia or neo-angiogenesis).
Early postnatal development represents a key transition in cardiac metabolism and complexity
The mammalian heart undergoes a marked increase in workload upon birth, and, in the mouse, the first few weeks of postnatal life are characterized by extensive ventricular remodeling coincident with switch from anaerobic metabolism (glycolysis) to aerobic metabolism (oxidative phosphorylation of fatty acids)99,105. Experiments performed in rabbits show that while circulating levels of fatty acid are high following birth, the switch from glycolysis to aerobic metabolism occurs only at the end of the first week105 and the neonate heart retains an enriched ability to produce ATP by glycolytic metabolism75 (Figure 2). Postnatal myocardium growth involves an increase in hemodynamic demands and is characterized by a thickening and vascularization of the ventricular myocardial wall27,106–108. Mouse postnatal growth follows three main steps: hyperplasia (postnatal day 0 [P0] to P4), the transitional phase when hyperplasia and hypertrophy processes occur simultaneously (P5 to P15), and hypertrophy (after P15)107,109,110. Studies in rodent models have shown that a last round of DNA synthesis and karyokinesis takes place without cytokinesis during the hypertrophy phase, culminating in the bi-nucleation of postnatal cardiomyocytes107,109,111–114. In addition to cardiomyocyte maturation, there is a marked growth and maturation of the coronary vasculature115. The perinatal period is characterized by angiogenesis and an expansion of the capillary network115,116. This increase of the coronary tree is due to the proliferation of pre-existing capillary endothelial cells and increase of the capillary length116 as well as increases in the thickness, length, and branching of arterial and venous coronary vasculature115,117,118.
Neonatal heart regeneration
In 2011, Porrello and colleagues119 demonstrated that the mouse neonatal heart regenerates in response to ventricular apex resection as well as experimentally induced myocardial infarction (MI)120. The regenerative process of the neonate heart is characterized by clot formation at the site of injury coupled with an inflammatory response followed by epicardial cell and cardiomyocyte proliferation, ultimately leading to a restoration of cardiac function119,120. It is of interest that this regenerative capacity is lost during the first few days after birth119,121, which, as noted above, corresponds to the time point during which the terminal differentiation of cardiomyocytes is concluded109,111. Although these studies support the proposal that the mammalian neonatal heart possesses pronounced regenerative capacity, including the ability to replace cardiomyocytes, these results have been challenged by using the same experimental model in which limited cardiomyocyte proliferation and deficient neo-angiogenesis coupled with extensive scarring were observed122,123. These different outcomes have been suggested to be due to technical variation123–126, difficulties in tracking mouse cardiomyocyte proliferation during the first week of life127, and timing of post-injury follow-up, where longer post-injury period allows more time for scar tissue deposition128. Although there remains considerable debate regarding the efficiency of neonatal heart regeneration, it is generally agreed that the neonatal myocardium has some proliferative and angiogenic capacity which is lost in the adult heart128. What might explain this more robust repair capacity during the first week of postnatal life in the mouse? As outlined in the previous section, the murine heart undergoes a metabolic switch to oxidative metabolism coupled with the maturation and bi-nucleation of cardiomyocytes, and the maturation of the coronary vasculature, which is driven by the availability of oxygen. This raises the hypothesis that low levels of oxygen during early postnatal life present a permissive context in which regeneration can occur.
Tissue repair in the adult heart: adult cardiac progenitor cells
The adult mammalian heart is one of the least-regenerative organs in the body; thus, injury, most notably MI, leads to a progressive decrease in heart function and ultimately results in heart failure. In the past two decades, several studies have shown that the uninjured adult heart replaces a low percentage of the total cardiomyocytes129–131. Renewal of pre-existing cardiomyocytes is approximately 0.5% to 1% per year, a rate that declines with age129. This low turnover rate implies that the vast majority of cardiomyocytes present at the conclusion of postnatal development remain throughout the entirety of adult life129,132. It has been demonstrated that adult cardiomyocytes undergo a fourfold increase in turnover following injury129,132–135; however, the origin of new cardiomyocytes remains unclear. Several studies have provided evidence for the existence of adult resident CPCs that are able to give rise to cardiomyocytes and non-myocytes that represent a potential source for heart regeneration (Table 1). CPCs have been defined and isolated by the expression of different markers clustered in niches in specific regions such as the atria, apex, and epicardium58,136,137 (Table 1); however, it is still unclear whether these different subsets belong to the same cell population or represent different CPC populations or do both. Additionally, the developmental origin of adult CPCs remains largely unknown, except for some of the CPC subsets that maintain a protein signature highly related with the embryonic populations such as the PEO-derived138–140 and Isl1+141–143 subsets (Table 1). After injury, CPCs are activated and give rise to different cell types (that is, myofibroblasts and smooth muscle cells and, to a lesser extent, endothelial cells and cardiomyocytes)144. Of note, the contribution of these cells to new cardiomyocytes during steady-state and injury is highly debated145. One high-profile example is the c-kit expressing CPCs, which have been reported to possess proliferative capacity and the ability to differentiate into cardiomyocytes146–149,171, whereas other studies have refuted these observations and observe little to no cardiomyogenic potential172–175. Other studies have provided evidence demonstrating that the formation of new cardiomyocytes comes from pre-existing cardiomyocytes that proliferate in human steady-state young heart as well as in pathological conditions129,135,176–178. Similarly, in the mouse, studies have suggested that new cardiomyocytes are derived from pre-existing cardiomyocytes that re-enter the cell cycle120,135,172,179–182 or arise from dedifferentiated myocytes183,184. Regardless of whether one or both of these models are accurate, it is clear that the adult heart is unable to functionally restore the adult myocardium following cardiac injury129,135,145,185,186. In addition to the inefficient renewal of cardiomyocytes after injury, little revascularization is observed in the adult heart following injury, which likely contributes to poor cardiac regeneration187,188. Some studies revealed that endocardial cells, resident endothelial cells, or even fibroblasts can adopt an endothelial cell-like fate and contribute to angiogenesis in response to cardiac injury; however, the involvement of CPCs in this process remains largely controversial189–191. In the next section, we consider whether the potential of the heart to neovascularize following injury is a key process for future therapeutic targeting for heart disease.
Table 1. Different identified adult cardiac progenitor cell populations.
| Adult cardiac progenitor cells | Embryonic
origin | Progenies | References |
|---|
| Identification/isolation by cell surface markers |
| c-kit+ | Unknown | Cardiomyocytes, endothelial cells, and
smooth muscle cells | 146–149,150 |
| Sca-1+ | Unknown | Cardiomyocytes, endothelial cells, smooth
muscle cells, and fibroblasts | 151–156 |
| Islet-1+ | SHF proposed | Smooth muscle cells, endothelial cells,
parasympathetic neurons, sinoatrial node
cells, and cardiomyocytes | 20,141–143,157,158 |
Platelet-derived growth factor
receptor alpha+ (PDGFRα+) | Unknown | Smooth muscle cells and endothelial cells | 159,160 |
| Mesoangioblasts | Unknown | Cardiomyocytes | 161,162 |
Epicardial-derived progenitor
cells | PEO | Coronary smooth muscle cells and adventitial
and interstitial fibroblasts | 56,138,140,163 |
| Identification by dye-efflux function |
| Side population | Unknown | Cardiomyocytes, endothelial cells, smooth
muscle cells, and fibroblasts | 151,164–167 |
| Isolation by in vitro assays |
Cardiospheres and
cardiosphere-derived cells | Unknown | Cardiomyocytes | 168–170 |
Cardiac-resident colony-forming
units-fibroblasts (cCFU-Fs) | PEO proposed | Fibroblasts | 139 |
The role of the adult epicardium in cardiac repair
The adult myocardium is surrounded and protected by the epicardium, which consists of a single mesothelial cell layer. As described in the previous section, the fetal epicardium is a source of cellular components and paracrine signals that promote coronary vasculature formation and myocardial growth192–196. Once the heart is mature, EPDCs progressively lose their capacity to undergo EMT and the epicardium enters a primarily quiescent state195,197,198 (Figure 3). In the adult, the role of the epicardium is to provide a physical barrier against infection199. More recently, it has been shown that the epicardium is a key responder to heart injury195,198,200 (Figure 3). Specifically, the epicardium re-expresses genes typically observed during fetal epicardial development in response to injury, including Wt1, Tbx18, and Raldh2, coupled with proliferation and EMT to form a thick layer of EPDC mesenchymal cells163,195. EPDCs contribute to adventitial and interstitial fibroblasts and smooth muscle cells195,201,202 (Figure 3), suggesting that the adult epicardium serves as a reservoir for mesenchymal progenitor cells203.
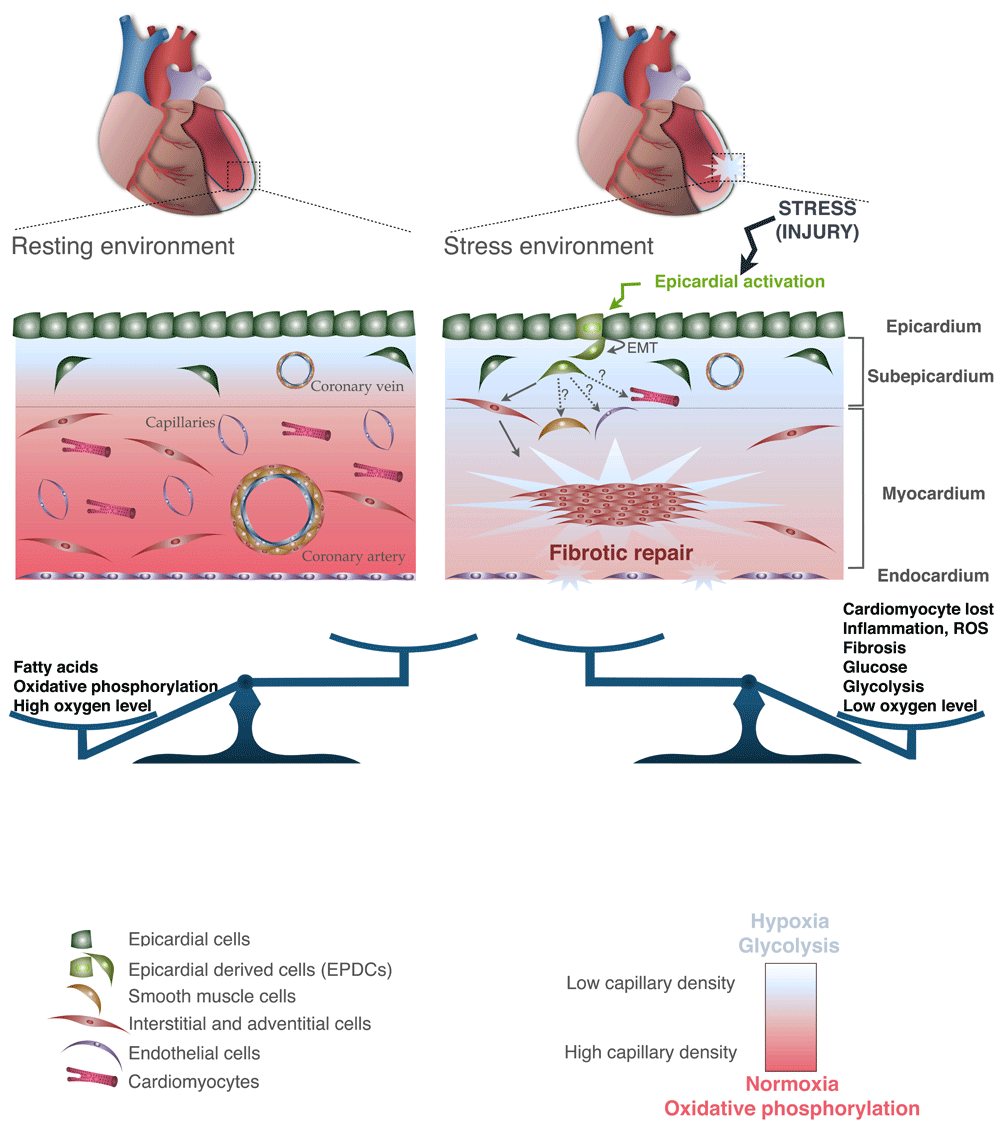
Figure 3. Adult heart under physiological conditions and after injury.
Both the microenvironment and the cellular component are represented. A color code was assigned and followed to define each of the cardiac cell types and their associated cells (depicted in the legend). EMT, epithelial-to-mesenchymal transition.
It has been shown that thymosin β-4 (Tβ-4) treatment results in adult epicardial activation, migration, and differentiation of EPDCs into smooth muscle cells, endothelial cells, and fibroblasts58,204. This is of interest since Tβ-4 is a key regulator of angiogenesis that acts through the stimulation of Vegf and stabilizing the hypoxia-inducible factor 1α (Hif-1α)205. It has been shown that Tβ-4 is expressed by the developing myocardium and regulates coronary vessel development (vasculogenesis, angiogenesis, and arteriogenesis) through activation of the epicardium58,205. Conversely, Banerjee and colleagues206 could not confirm these results and claimed there is no link between Tβ-4 and angiogenesis during heart morphogenesis. The different transgenic models used in the two studies may explain the discrepancy of the results obtained. Tβ-4 is also able to promote the vascular potential of adult EPDCs, which contributes to an enhanced cardiomyocyte survival after injury58,163,205,207. Irrespective of the angiogenic properties of Tβ-4 during development, additional studies emphasize the involvement of epicardial cells and EPDCs in vasculature formation after cardiac injury. Wagner and colleagues208 demonstrated that Wt1, the epicardial master transcription factor, is expressed in the coronary vessels of injured heart, suggesting a role for Wt1 in the vascular growth after cardiac injury. Additionally, proangiogenic factors, such as VEGF and FGF, are secreted by EPDCs during the cardiac repair process, promoting the growth and survival of the heart vasculature195.
In addition to providing a protective role for the heart, the epicardium serves as a reservoir for CPCs that are activated in response to injury. As such, the adult epicardium merits future research for targeting and ameliorating mammalian heart repair.
The adult cardiac cellular microenvironment
The heart continually adapts to changing workloads brought about by aging, physical activities, and disease. The heart produces energy using multiple metabolic substrates such as fatty acids, glucose, ketone bodies, lactate, and amino acids209–213. It is estimated that the heart uses between 3.5 and 5 kg of ATP every day210. Fatty acids are more energy-dense and thus provide more ATP molecules per consumed carbon as compared with the other substrates; however, fatty acid pathway requires more oxygen210,211,213. A healthy heart uses primarily long-chain fatty acids, which provide 60% to 70% of the produced ATP to power the muscle contraction, while the remaining energy is derived from carbohydrate metabolism209–211,213,214. To meet this high ATP demand, the heart requires oxygen. When the body is at rest, myocardial oxygen consumption is greater than the oxygen consumption of any other organ of the body210,211,215. Coronary circulation ensures the delivery of metabolic substrates and oxygen to the myocardium. Under physiological conditions, 90% of the ATP is produced through mitochondrial oxidative phosphorylation (fatty acids and glucose), which is an oxygen-dependent process216. However, hypoxic conditions resulting from either physiological states (for example, during exercise) or pathological states (for example, coronary artery disease with mild ischemia) require the glycolytic pathway in order to produce ATP210,211,217.
Although the adult heart is highly reliant on the availability of high levels of oxygen, the ventricles have a non-uniform distribution of oxygen across the wall with an oxygen tension gradient: lower tension in the extremities—that is, subendocardial (pO2 = 10 mmHg) and subepicardial (pO2 = 18 mmHg) regions—and higher tension in the middle of the myocardium (pO2 = 38 mmHg)218. Reflecting the different levels of oxygen tension in the heart, Hif-1α, known to be stable under hypoxic conditions, is detected at high levels in the epicardium and subepicardium219,220. Although the high levels of environmental oxygen and adult stem cell niches (for example, bone marrow221–223) are characterized by low concentration of oxygen tension, this favors glycolytic metabolism. Although it has been shown to be essential, the exact mechanism of glycolytic metabolism and hypoxia in the maintenance of stemness properties is not well understood219,223. A protective mechanism of the adult stem cells against reactive oxygen species (ROS) has been implicit, but more studies are needed180,181,219. As described previously, the epicardium represents a reservoir of adult CPCs and, like other adult stem cell niches, displays a low oxygen tension and relies on glycolysis-dependent metabolic pathways (cytoplasmic glycolysis)219,224–226 (Figure 3).
Potential advantages of environmental manipulation to stimulate epicardial cells to a more efficient repair
Cardiovascular diseases are a major cause of human mortality227. At present, long-term and effective treatments are missing. It is noteworthy that in high-altitude regions, cardiovascular diseases are less prevalent228–230, which has been attributed to continuous low-level hypoxia103,231,232. Hypoxia activates an evolutionarily conserved adaptive process that allows mammals to cope with restricted oxygen tension. Indeed, as already described in this review, the hypoxic environment promotes a metabolic switch from aerobic mitochondrial metabolism to anaerobic cytoplasmic glycolysis, which is an essential feature of heart development driving cell proliferation, self-renewal, and differentiation78,80–83 as well as of specific cardiac processes such as ventricular wall expansion through cardiomyocyte hyperplasia28,30,83 or stimulation of angiogenesis and formation of coronary vasculature84–87. This feature of adult progenitor cells allows ATP production in the absence of oxygen, providing an advantage in a hypoxic environment219. Additionally, it has become clearer that while oxidative metabolism is more efficient for producing ATP, cellular senescence and cell cycle arrest are a consequence of the resultant oxidative stress180.
Although this review is focused on mammalian heart biology, it is important to note that lower vertebrates such as the zebrafish maintain a capacity to fully regenerate large domains of damaged myocardium through the proliferation of pre-existing cardiomyocytes233,234, which is similarly triggered by a hypoxic protective response235. These results prompted Sadek and colleagues236,237 to explore the response of the mammalian heart to injury under hypoxic environmental conditions. Myocardial infarcted mice exposed for two weeks to hypoxia (7% oxygen) displayed a reduction of myocardial fibrosis and improved ventricular function. Sadek and colleagues proposed that the gradual reduction of environmental oxygen promotes glycolytic metabolism and reduces oxidative phosphorylation. This metabolic switch decreases ROS and cellular senescence and promotes the activation of proliferation of a small fraction of pre-existing cardiomyocytes236,237; however, it is also likely that additional progenitor populations contribute to this response. Additionally, Sadek and colleagues noted that there is a marked cardiac vascular network expansion (that is, the increase of coronary collaterals and capillary size) in the infarcted area after exposure to low levels of oxygen as compared with controls236. As discussed above, the angiogenesis is dependent upon the oxygen tension and thus on the metabolic status of the microenvironment.
Emerging evidence suggests that the epicardium is an important source of stromal progenitor cells during development and that it maintains this capacity in the adult, potentially because of the particular hypoxic and glycolytic environment preserved in this adult heart compartment. While chronic exposure to high altitude carries collateral health risks in humans, it is tempting to propose that low oxygen tension can maintain the epicardium in an activated state, thus preserving perinatal plasticity, which in turn leads to improved cardiac repair. Further investigation of the adult CPCs and their microenvironment will provide important leads for improving cardiac repair following injury.
Taken altogether, while robust heart regeneration in the adult has yet to be achieved through therapeutic intervention, an understanding of the environmental clues and metabolic state of the developing heart will provide essential clues for novel therapeutic approaches. In the short term, it may be important to reconsider methodologies used during patient recovery following heart injury or insufficiency or both. Specifically, a more efficient angiogenic repair coupled with reduced scar tissue formation may result from promoting a more “embryonic-like” cardiac tissue environment during the repair process. How this can be achieved in a clinical setting and what additional clues can be translated to the clinic will be an interesting focus for the next decade.
Authors’ contributions
AS and MV contributed to original draft preparation and to reviewing and editing. DS contributed to conceptualization and to reviewing and editing.
Grant information
The laboratory is supported by the Laboratoire d’Excellence Revive (Investissement d’Avenir, ANR-10-LABX-73), the Fondation Leducq (grant 13CVD01; CardioStemNet project), and Agence Nationale pour la Recherche (ANR) grant RHU CARMMA (ANR-15-RHUS-0003).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Faculty Opinions recommendedReferences
- 1.
Nag AC:
Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution.
Cytobios.
1980; 28(109): 41–61. PubMed Abstract
- 2.
Banerjee I, Yekkala K, Borg TK, et al.:
Dynamic interactions between myocytes, fibroblasts, and extracellular matrix.
Ann N Y Acad Sci.
2006; 1080: 76–84. PubMed Abstract
| Publisher Full Text
- 3.
Saga Y, Miyagawa-Tomita S, Takagi A, et al.:
MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube.
Development.
1999; 126(15): 3437–3447. PubMed Abstract
- 4.
Kitajima T, Nishii K, Ueoka H, et al.:
Recent improvement in lung cancer screening: a comparison of the results carried out in two different time periods.
Acta Med Okayama.
2006; 60(3): 173–179. PubMed Abstract
| Publisher Full Text
- 5.
Jiang X, Rowitch DH, Soriano P, et al.:
Fate of the mammalian cardiac neural crest.
Development.
2000; 127(8): 1607–1616. PubMed Abstract
- 6.
Virágh S, Challice CE:
The origin of the epicardium and the embryonic myocardial circulation in the mouse.
Anat Rec.
1981; 201(1): 157–168. PubMed Abstract
| Publisher Full Text
- 7.
Pérez-Pomares JM, Phelps A, Sedmerova M, et al.:
Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium.
Dev Dyn.
2003; 227(1): 56–68. PubMed Abstract
| Publisher Full Text
- 8.
Wu SM, Fujiwara Y, Cibulsky SM, et al.:
Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart.
Cell.
2006; 127(6): 1137–1150. PubMed Abstract
| Publisher Full Text
- 9.
Meilhac SM, Esner M, Kelly RG, et al.:
The clonal origin of myocardial cells in different regions of the embryonic mouse heart.
Dev Cell.
2004; 6(5): 685–698. PubMed Abstract
| Publisher Full Text
- 10.
Saga Y, Kitajima S, Miyagawa-Tomita S:
Mesp1 expression is the earliest sign of cardiovascular development.
Trends Cardiovasc Med.
2000; 10(8): 345–352. PubMed Abstract
| Publisher Full Text
- 11.
Harvey RP:
Patterning the vertebrate heart.
Nat Rev Genet.
2002; 3(7): 544–556. PubMed Abstract
| Publisher Full Text
- 12.
Zaffran S, Kelly RG, Meilhac SM, et al.:
Right ventricular myocardium derives from the anterior heart field.
Circ Res.
2004; 95(3): 261–268. PubMed Abstract
| Publisher Full Text
- 13.
Nishii K, Shibata Y:
Mode and determination of the initial contraction stage in the mouse embryo heart.
Anat Embryol (Berl).
2006; 211(2): 95–100. PubMed Abstract
| Publisher Full Text
- 14.
Navaratnam V, Kaufman MH, Skepper JN, et al.:
Differentiation of the myocardial rudiment of mouse embryos: an ultrastructural study including freeze-fracture replication.
J Anat.
1986; 146: 65–85. PubMed Abstract
| Free Full Text
- 15.
Kelly RG, Brown NA, Buckingham ME:
The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm.
Dev Cell.
2001; 1(3): 435–440. PubMed Abstract
| Publisher Full Text
- 16.
Cai CL, Liang X, Shi Y, et al.:
Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart.
Dev Cell.
2003; 5(6): 877–889. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 17.
Galli D, Domínguez JN, Zaffran S, et al.:
Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed.
Development.
2008; 135(6): 1157–1167. PubMed Abstract
| Publisher Full Text
- 18.
Verzi MP, McCulley DJ, De Val S, et al.:
The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field.
Dev Biol.
2005; 287(1): 134–145. PubMed Abstract
| Publisher Full Text
- 19.
Buckingham M, Meilhac S, Zaffran S:
Building the mammalian heart from two sources of myocardial cells.
Nat Rev Genet.
2005; 6(11): 826–835. PubMed Abstract
| Publisher Full Text
- 20.
Moretti A, Caron L, Nakano A, et al.:
Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification.
Cell.
2006; 127(6): 1151–1165. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 21.
Sun Y, Liang X, Najafi N, et al.:
Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells.
Dev Biol.
2007; 304(1): 286–296. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Watanabe Y, Buckingham M:
The formation of the embryonic mouse heart: heart fields and myocardial cell lineages.
Ann N Y Acad Sci.
2010; 1188(1): 15–24. PubMed Abstract
| Publisher Full Text
- 23.
Waldo KL, Hutson MR, Ward CC, et al.:
Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart.
Dev Biol.
2005; 281(1): 78–90. PubMed Abstract
| Publisher Full Text
- 24.
Harmon AW, Nakano A:
Nkx2-5 lineage tracing visualizes the distribution of second heart field-derived aortic smooth muscle.
Genesis.
2013; 51(12): 862–869. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Ma Q, Zhou B, Pu WT:
Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity.
Dev Biol.
2008; 323(1): 98–104. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Minot CS:
On a Hitherto Unrecognized Form of Blood Circulation without Capillaries in the Organs of Vertebrates.
J Boston Soc Med Sci.
1900; 4(6): 133–134. PubMed Abstract
| Free Full Text
- 27.
Sedmera D, Pexieder T, Vuillemin M:
Developmental patterning of the myocardium.
Anat Rec.
2000; 258(4): 319–337. PubMed Abstract
| Publisher Full Text
- 28.
Sedmera D, Reckova M, DeAlmeida A, et al.:
Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system.
Anat Rec A Discov Mol Cell Evol Biol.
2003; 274(1): 773–777. PubMed Abstract
| Publisher Full Text
- 29.
Sedmera D, Thompson RP:
Myocyte proliferation in the developing heart.
Dev Dyn.
2011; 240(6): 1322–1334. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
de Boer BA, van den Berg G, de Boer PA, et al.:
Growth of the developing mouse heart: an interactive qualitative and quantitative 3D atlas.
Dev Biol.
2012; 368(2): 203–213. PubMed Abstract
| Publisher Full Text
- 31.
Pérez-Pomares JM, Phelps A, Sedmerova M, et al.:
Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs).
Dev Biol.
2002; 247(2): 307–326. PubMed Abstract
| Publisher Full Text
- 32.
Chen T, Chang TC, Kang JO, et al.:
Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor.
Dev Biol.
2002; 250(1): 198–207. PubMed Abstract
| Publisher Full Text
- 33.
Pennisi DJ, Ballard VL, Mikawa T:
Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart.
Dev Dyn.
2003; 228(2): 161–172. PubMed Abstract
| Publisher Full Text
- 34.
Baldwin HS:
Early embryonic vascular development.
Cardiovasc Res.
1996; 31 Spec No: E34–45. PubMed Abstract
| Publisher Full Text
- 35.
Drake CJ, Fleming PA:
Vasculogenesis in the day 6.5 to 9.5 mouse embryo.
Blood.
2000; 95(5): 1671–1679. PubMed Abstract
- 36.
Misfeldt AM, Boyle SC, Tompkins KL, et al.:
Endocardial cells are a distinct endothelial lineage derived from Flk1+ multipotent cardiovascular progenitors.
Dev Biol.
2009; 333(1): 78–89. PubMed Abstract
| Publisher Full Text
- 37.
Gassmann M, Casagranda F, Orioli D, et al.:
Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor.
Nature.
1995; 378(6555): 390–394. PubMed Abstract
| Publisher Full Text
- 38.
Lee KF, Simon H, Chen H, et al.:
Requirement for neuregulin receptor erbB2 in neural and cardiac development.
Nature.
1995; 378(6555): 394–398. PubMed Abstract
| Publisher Full Text
- 39.
Wagner M, Siddiqui MA:
Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation.
Exp Biol Med (Maywood).
2007; 232(7): 866–880. PubMed Abstract
- 40.
Stankunas K, Hang CT, Tsun ZY, et al.:
Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis.
Dev Cell.
2008; 14(2): 298–311. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 41.
Rychterová V:
Principle of growth in thickness of the heart ventricular wall in the chick embryo.
Folia Morphol (Praha).
1971; 19(3): 262–272. PubMed Abstract
- 42.
Sedmera D, Pexieder T, Hu N, et al.:
Developmental changes in the myocardial architecture of the chick.
Anat Rec.
1997; 248(3): 421–432. PubMed Abstract
| Publisher Full Text
- 43.
Wessels A, Sedmera D:
Developmental anatomy of the heart: a tale of mice and man.
Physiol Genomics.
2003; 15(3): 165–176. PubMed Abstract
| Publisher Full Text
- 44.
Wu B, Zhang Z, Lui W, et al.:
Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling.
Cell.
2012; 151(5): 1083–1096. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 45.
Tian X, Hu T, Zhang H, et al.:
Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart.
Science.
2014; 345(6192): 90–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 46.
Combs MD, Yutzey KE:
Heart valve development: regulatory networks in development and disease.
Circ Res.
2009; 105(5): 408–421. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Ranger AM, Grusby MJ, Hodge MR, et al.:
The transcription factor NF-ATc is essential for cardiac valve formation.
Nature.
1998; 392(6672): 186–190. PubMed Abstract
| Publisher Full Text
- 48.
Person AD, Klewer SE, Runyan RB:
Cell biology of cardiac cushion development.
Int Rev Cytol.
2005; 243: 287–335. PubMed Abstract
| Publisher Full Text
- 49.
Snarr BS, Kern CB, Wessels A:
Origin and fate of cardiac mesenchyme.
Dev Dyn.
2008; 237(10): 2804–2819. PubMed Abstract
| Publisher Full Text
- 50.
Eisenberg LM, Markwald RR:
Molecular regulation of atrioventricular valvuloseptal morphogenesis.
Circ Res.
1995; 77(1): 1–6. PubMed Abstract
| Publisher Full Text
- 51.
de Lange FJ, Moorman AF, Anderson RH, et al.:
Lineage and morphogenetic analysis of the cardiac valves.
Circ Res.
2004; 95(6): 645–654. PubMed Abstract
| Publisher Full Text
- 52.
Wu B, Wang Y, Lui W, et al.:
Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation.
Circ Res.
2011; 109(2): 183–192. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 53.
Kirby ML, Waldo KL:
Neural crest and cardiovascular patterning.
Circ Res.
1995; 77(2): 211–215. PubMed Abstract
- 54.
Schulte I, Schlueter J, Abu-Issa R, et al.:
Morphological and molecular left-right asymmetries in the development of the proepicardium: a comparative analysis on mouse and chick embryos.
Dev Dyn.
2007; 236(3): 684–695. PubMed Abstract
| Publisher Full Text
- 55.
van Wijk B, van den Berg G, Abu-Issa R, et al.:
Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways.
Circ Res.
2009; 105(5): 431–441. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 56.
Wessels A, Pérez-Pomares JM:
The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells.
Anat Rec A Discov Mol Cell Evol Biol.
2004; 276(1): 43–57. PubMed Abstract
| Publisher Full Text
- 57.
Pérez-Pomares JM, Carmona R, González-Iriarte M, et al.:
Origin of coronary endothelial cells from epicardial mesothelium in avian embryos.
Int J Dev Biol.
2002; 46(8): 1005–1013. PubMed Abstract
- 58.
Smart N, Risebro CA, Melville AA, et al.:
Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization.
Nature.
2007; 445(7124): 177–182. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 59.
Zhou B, Ma Q, Rajagopal S, et al.:
Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart.
Nature.
2008; 454(7200): 109–113. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 60.
Cai CL, Martin JC, Sun Y, et al.:
A myocardial lineage derives from Tbx18 epicardial cells.
Nature.
2008; 454(7200): 104–108. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 61.
Tian X, Hu T, Zhang H, et al.:
Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries.
Cell Res.
2013; 23(9): 1075–1090. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 62.
Christoffels VM, Grieskamp T, Norden J, et al.:
Tbx18 and the fate of epicardial progenitors.
Nature.
2009; 458(7240): E8–9; discussion E9–10. PubMed Abstract
| Publisher Full Text
- 63.
Tian X, Pu WT, Zhou B:
Cellular origin and developmental program of coronary angiogenesis.
Circ Res.
2015; 116(3): 515–530. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 64.
Tomanek RJ, Ishii Y, Holifield JS, et al.:
VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo.
Circ Res.
2006; 98(7): 947–953. PubMed Abstract
| Publisher Full Text
- 65.
Kwee L, Burns DK, Rumberger JM, et al.:
Creation and characterization of E-selectin- and VCAM-1-deficient mice.
Ciba Found Symp.
1995; 189: 17–28; discussion 28–34, 77–18. PubMed Abstract
| Publisher Full Text
- 66.
Mohan RA, Boukens BJ, Christoffels VM:
Developmental Origin of the Cardiac Conduction System: Insight from Lineage Tracing.
Pediatr Cardiol.
2018; 39(6): 1107–1114. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 67.
Mommersteeg MT, Domínguez JN, Wiese C, et al.:
The sinus venosus progenitors separate and diversify from the first and second heart fields early in development.
Cardiovasc Res.
2010; 87(1): 92–101. PubMed Abstract
| Publisher Full Text
- 68.
Miquerol L, Moreno-Rascon N, Beyer S, et al.:
Biphasic development of the mammalian ventricular conduction system.
Circ Res.
2010; 107(1): 153–161. PubMed Abstract
| Publisher Full Text
- 69.
Miquerol L, Beyer S, Kelly RG:
Establishment of the mouse ventricular conduction system.
Cardiovasc Res.
2011; 91(2): 232–242. PubMed Abstract
| Publisher Full Text
- 70.
Aanhaanen WT, Mommersteeg MT, Norden J, et al.:
Developmental origin, growth, and three-dimensional architecture of the atrioventricular conduction axis of the mouse heart.
Circ Res.
2010; 107(6): 728–736. PubMed Abstract
| Publisher Full Text
- 71.
Nakamura T, Colbert MC, Robbins J:
Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system.
Circ Res.
2006; 98(12): 1547–1554. PubMed Abstract
| Publisher Full Text
- 72.
Lescroart F, Meilhac SM:
Cell lineages, growth and repair of the mouse heart.
Results Probl Cell Differ.
2012; 55: 263–289. PubMed Abstract
| Publisher Full Text
- 73.
Patterson AJ, Zhang L:
Hypoxia and fetal heart development.
Curr Mol Med.
2010; 10(7): 653–666. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 74.
Fisher DJ:
Oxygenation and metabolism in the developing heart.
Semin Perinatol.
1984; 8(3): 217–225. PubMed Abstract
- 75.
Ascuitto RJ, Ross-Ascuitto NT:
Substrate metabolism in the developing heart.
Semin Perinatol.
1996; 20(6): 542–563. PubMed Abstract
| Publisher Full Text
- 76.
Nanka O, Valásek P, Dvoráková M, et al.:
Experimental hypoxia and embryonic angiogenesis.
Dev Dyn.
2006; 235(3): 723–733. PubMed Abstract
| Publisher Full Text
- 77.
Wikenheiser J, Doughman YQ, Fisher SA, et al.:
Differential levels of tissue hypoxia in the developing chicken heart.
Dev Dyn.
2006; 235(1): 115–123. PubMed Abstract
| Publisher Full Text
- 78.
Giaccia AJ, Simon MC, Johnson R:
The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease.
Genes Dev.
2004; 18(18): 2183–2194. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 79.
Sharma SK, Lucitti JL, Nordman C, et al.:
Impact of hypoxia on early chick embryo growth and cardiovascular function.
Pediatr Res.
2006; 59(1): 116–120. PubMed Abstract
| Publisher Full Text
- 80.
Compernolle V, Brusselmans K, Franco D, et al.:
Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha.
Cardiovasc Res.
2003; 60(3): 569–579. PubMed Abstract
| Publisher Full Text
- 81.
Sugishita Y, Leifer DW, Agani F, et al.:
Hypoxia-responsive signaling regulates the apoptosis-dependent remodeling of the embryonic avian cardiac outflow tract.
Dev Biol.
2004; 273(2): 285–296. PubMed Abstract
| Publisher Full Text
- 82.
Dunwoodie SL:
The role of hypoxia in development of the Mammalian embryo.
Dev Cell.
2009; 17(6): 755–773. PubMed Abstract
| Publisher Full Text
- 83.
Guimarães-Camboa N, Stowe J, Aneas I, et al.:
HIF1α Represses Cell Stress Pathways to Allow Proliferation of Hypoxic Fetal Cardiomyocytes.
Dev Cell.
2015; 33(5): 507–521. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 84.
Tomanek RJ, Lotun K, Clark EB, et al.:
VEGF and bFGF stimulate myocardial vascularization in embryonic chick.
Am J Physiol.
1998; 274(5 Pt 2): H1620–1626. PubMed Abstract
| Publisher Full Text
- 85.
Edelberg JM, Aird WC, Wu W, et al.:
PDGF mediates cardiac microvascular communication.
J Clin Invest.
1998; 102(4): 837–843. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 86.
Tomanek RJ, Ratajska A, Kitten GT, et al.:
Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis.
Dev Dyn.
1999; 215(1): 54–61. PubMed Abstract
| Publisher Full Text
- 87.
Tomanek RJ, Lund DD, Yue X:
Hypoxic induction of myocardial vascularization during development.
Adv Exp Med Biol.
2003; 543: 139–149. PubMed Abstract
| Publisher Full Text
- 88.
Jansen CA, Krane EJ, Thomas AL, et al.:
Continuous variability of fetal PO2 in the chronically catheterized fetal sheep.
Am J Obstet Gynecol.
1979; 134(7): 776–783. PubMed Abstract
| Publisher Full Text
- 89.
Jensen A, Garnier Y, Berger R:
Dynamics of fetal circulatory responses to hypoxia and asphyxia.
Eur J Obstet Gynecol Reprod Biol.
1999; 84(2): 155–172. PubMed Abstract
| Publisher Full Text
- 90.
Myatt L:
Placental adaptive responses and fetal programming.
J Physiol.
2006; 572(Pt 1): 25–30. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 91.
Reynolds JD, Penning DH, Dexter F, et al.:
Ethanol increases uterine blood flow and fetal arterial blood oxygen tension in the near-term pregnant ewe.
Alcohol.
1996; 13(3): 251–256. PubMed Abstract
| Publisher Full Text
- 92.
Lawrence J, Xiao D, Xue Q, et al.:
Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring.
J Pharmacol Exp Ther.
2008; 324(1): 331–341. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 93.
Ream M, Ray AM, Chandra R, et al.:
Early fetal hypoxia leads to growth restriction and myocardial thinning.
Am J Physiol Regul Integr Comp Physiol.
2008; 295(2): R583–595. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 94.
Bae S, Xiao Y, Li G, et al.:
Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart.
Am J Physiol Heart Circ Physiol.
2003; 285(3): H983–990. PubMed Abstract
| Publisher Full Text
- 95.
Barker DJ:
The fetal and infant origins of adult disease.
BMJ.
1990; 301(6761): 1111. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 96.
Okamura T, Miura T, Takemura G, et al.:
Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart.
Cardiovasc Res.
2000; 45(3): 642–650. PubMed Abstract
| Publisher Full Text
- 97.
Kamitomo M, Onishi J, Gutierrez I, et al.:
Effects of long-term hypoxia and development on cardiac contractile proteins in fetal and adult sheep.
J Soc Gynecol Investig.
2002; 9(6): 335–341. PubMed Abstract
| Publisher Full Text
- 98.
Li G, Xiao Y, Estrella JL, et al.:
Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat.
J Soc Gynecol Investig.
2003; 10(5): 265–274. PubMed Abstract
| Publisher Full Text
- 99.
Scholz TD, Segar JL:
Cardiac Metabolism in the Fetus and Newborn.
NeoReviews.
2008; 9(3): e109–e118. Publisher Full Text
- 100.
Soothill PW, Nicolaides KH, Rodeck CH, et al.:
Blood gases and acid-base status of the human second-trimester fetus.
Obstet Gynecol.
1986; 68(2): 173–176. PubMed Abstract
| Publisher Full Text
- 101.
Iwamoto HS, Kaufman T, Keil LC, et al.:
Responses to acute hypoxemia in fetal sheep at 0.6-0.7 gestation.
Am J Physiol.
1989; 256(3 Pt 2): H613–620. PubMed Abstract
| Publisher Full Text
- 102.
Michiels C:
Physiological and pathological responses to hypoxia.
Am J Pathol.
2004; 164(6): 1875–1882. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 103.
Beall CM:
Two routes to functional adaptation: Tibetan and Andean high-altitude natives.
Proc Natl Acad Sci U S A.
2007; 104 Suppl 1: 8655–8660. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 104.
Adelman DM, Gertsenstein M, Nagy A, et al.:
Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses.
Genes Dev.
2000; 14(24): 3191–3203. PubMed Abstract
| Free Full Text
- 105.
Lopaschuk GD, Spafford MA, Marsh DR:
Glycolysis is predominant source of myocardial ATP production immediately after birth.
Am J Physiol.
1991; 261(6 Pt 2): H1698–1705. PubMed Abstract
| Publisher Full Text
- 106.
Oparil S, Bishop SP, Clubb FJ Jr:
Myocardial cell hypertrophy or hyperplasia.
Hypertension.
1984; 6(6 Pt 2): III38–43. PubMed Abstract
| Publisher Full Text
- 107.
Clubb FJ Jr, Bishop SP:
Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy.
Lab Invest.
1984; 50(5): 571–577. PubMed Abstract
- 108.
Clark LT:
Anatomic substrate differences between black and white victims of sudden cardiac death: hypertension, coronary artery disease, or both?
Clin Cardiol.
1989; 12(12 Suppl 4): IV13–17. PubMed Abstract
| Publisher Full Text
- 109.
Li F, Wang X, Capasso JM, et al.:
Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development.
J Mol Cell Cardiol.
1996; 28(8): 1737–1746. PubMed Abstract
| Publisher Full Text
- 110.
Leu M, Ehler E, Perriard JC:
Characterisation of postnatal growth of the murine heart.
Anat Embryol (Berl).
2001; 204(3): 217–224. PubMed Abstract
| Publisher Full Text
- 111.
Soonpaa MH, Kim KK, Pajak L, et al.:
Cardiomyocyte DNA synthesis and binucleation during murine development.
Am J Physiol.
1996; 271(5 Pt 2): H2183–2189. PubMed Abstract
| Publisher Full Text
- 112.
Li F, Wang X, Bunger PC, et al.:
Formation of binucleated cardiac myocytes in rat heart: I. Role of actin-myosin contractile ring.
J Mol Cell Cardiol.
1997; 29(6): 1541–1551. PubMed Abstract
| Publisher Full Text
- 113.
Li F, Wang X, Gerdes AM:
Formation of binucleated cardiac myocytes in rat heart: II. Cytoskeletal organisation.
J Mol Cell Cardiol.
1997; 29(6): 1553–1565. PubMed Abstract
| Publisher Full Text
- 114.
Walsh S, Pontén A, Fleischmann BK, et al.:
Cardiomyocyte cell cycle control and growth estimation in vivo--an analysis based on cardiomyocyte nuclei.
Cardiovasc Res.
2010; 86(3): 365–373. PubMed Abstract
| Publisher Full Text
- 115.
Tomanek RJ:
Formation of the coronary vasculature: a brief review.
Cardiovasc Res.
1996; 31 Spec No: E46–51. PubMed Abstract
| Publisher Full Text
- 116.
Olivetti G, Anversa P, Loud AV:
Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. II. Tissue composition, capillary growth, and sarcoplasmic alterations.
Circ Res.
1980; 46(4): 503–12. PubMed Abstract
| Publisher Full Text
- 117.
Reinecke P, Hort W:
[The growth of coronary artery branches in man under physiological conditions. Morphological studies of corrosion casts of the anterior interventricular branch of the coronary artery].
Z Kardiol.
1992; 81(2): 110–115. PubMed Abstract
- 118.
Matonoha P, Zechmeister A:
Structure of the coronary arteries during the prenatal period in man.
Funct Dev Morphol.
1992; 2(3): 209–212. PubMed Abstract
- 119.
Porrello ER, Mahmoud AI, Simpson E, et al.:
Transient regenerative potential of the neonatal mouse heart.
Science.
2011; 331(6020): 1078–1080. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 120.
Porrello ER, Mahmoud AI, Simpson E, et al.:
Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family.
Proc Natl Acad Sci U S A.
2013; 110(1): 187–192. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 121.
Notari M, Ventura-Rubio A, Bedford-Guaus SJ, et al.:
The local microenvironment limits the regenerative potential of the mouse neonatal heart.
Sci Adv.
2018; 4(5): eaao5553. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 122.
Andersen DC, Ganesalingam S, Jensen CH, et al.:
Do neonatal mouse hearts regenerate following heart apex resection?
Stem Cell Reports.
2014; 2(4): 406–413. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 123.
Andersen DC, Jensen CH, Sheikh SP:
Response to Sadek et al. and Kotlikoff et al.
Stem Cell Reports.
2014; 3(1): 3–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 124.
Kotlikoff MI, Hesse M, Fleischmann BK:
Comment on "Do neonatal mouse hearts regenerate following heart apex resection"?
Stem Cell Reports.
2014; 3(1): 2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 125.
Sadek HA, Martin JF, Takeuchi JK, et al.:
Multi-investigator letter on reproducibility of neonatal heart regeneration following apical resection.
Stem Cell Reports.
2014; 3(1): 1. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 126.
Bryant DM, O'Meara CC, Ho NN, et al.:
A systematic analysis of neonatal mouse heart regeneration after apical resection.
J Mol Cell Cardiol.
2015; 79: 315–318. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 127.
Zebrowski DC, Becker R, Engel FB:
Towards regenerating the mammalian heart: challenges in evaluating experimentally induced adult mammalian cardiomyocyte proliferation.
Am J Physiol Heart Circ Physiol.
2016; 310(9): H1045–1054. PubMed Abstract
| Publisher Full Text
- 128.
Sampaio-Pinto V, Rodrigues SC, Laundos TL, et al.:
Neonatal Apex Resection Triggers Cardiomyocyte Proliferation, Neovascularization and Functional Recovery Despite Local Fibrosis.
Stem Cell Reports.
2018; 10(3): 860–874. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 129.
Bergmann O, Bhardwaj RD, Bernard S, et al.:
Evidence for cardiomyocyte renewal in humans.
Science.
2009; 324(5923): 98–102. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 130.
Kajstura J, Urbanek K, Perl S, et al.:
Cardiomyogenesis in the adult human heart.
Circ Res.
2010; 107(2): 305–315. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 131.
Soonpaa MH, Rubart M, Field LJ:
Challenges measuring cardiomyocyte renewal.
Biochim Biophys Acta.
2013; 1833(4):799–803. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 132.
Bergmann O, Zdunek S, Felker A, et al.:
Dynamics of Cell Generation and Turnover in the Human Heart.
Cell.
2015; 161(7): 1566–1575. PubMed Abstract
| Publisher Full Text
- 133.
Rumyantsev PP:
Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration.
Int Rev Cytol.
1977; 51: 186–273. PubMed Abstract
| Publisher Full Text
- 134.
Soonpaa MH, Field LJ:
Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts.
Am J Physiol.
1997; 272(1 Pt 2): H220–226. PubMed Abstract
| Publisher Full Text
- 135.
Senyo SE, Steinhauser ML, Pizzimenti CL, et al.:
Mammalian heart renewal by pre-existing cardiomyocytes.
Nature.
2013; 493(7432): 433–436. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 136.
Itzhaki-Alfia A, Leor J, Raanani E, et al.:
Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells.
Circulation.
2009; 120(25): 2559–2566. PubMed Abstract
| Publisher Full Text
- 137.
Leinonen JV, Emanuelov AK, Platt Y, et al.:
Left atrial appendages from adult hearts contain a reservoir of diverse cardiac progenitor cells.
PLoS One.
2013; 8(3): e59228. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 138.
Winter EM, Gittenberger-de Groot AC:
Epicardium-derived cells in cardiogenesis and cardiac regeneration.
Cell Mol Life Sci.
2007; 64(6): 692–703. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 139.
Chong JJ, Chandrakanthan V, Xaymardan M, et al.:
Adult cardiac-resident MSC-like stem cells with a proepicardial origin.
Cell Stem Cell.
2011; 9(6): 527–540. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 140.
Acharya A, Baek ST, Huang G, et al.:
The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors.
Development.
2012; 139(12): 2139–2149. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 141.
Laugwitz KL, Moretti A, Lam J, et al.:
Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages.
Nature.
2005; 433(7026): 647–653. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 142.
Bu L, Jiang X, Martin-Puig S, et al.:
Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages.
Nature.
2009; 460(7251): 113–117. PubMed Abstract
| Publisher Full Text
- 143.
Genead R, Danielsson C, Andersson AB, et al.:
Islet-1 cells are cardiac progenitors present during the entire lifespan: from the embryonic stage to adulthood.
Stem Cells Dev.
2010; 19(10): 1601–1615. PubMed Abstract
| Publisher Full Text
- 144.
Le T, Chong J:
Cardiac progenitor cells for heart repair.
Cell Death Discov.
2016; 2: 16052. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 145.
Valente M, Nascimento DS, Cumano A, et al.:
Sca-1+ cardiac progenitor cells and heart-making: a critical synopsis.
Stem Cells Dev.
2014; 23(19): 2263–2273. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 146.
Beltrami AP, Barlucchi L, Torella D, et al.:
Adult cardiac stem cells are multipotent and support myocardial regeneration.
Cell.
2003; 114(6): 763–776. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 147.
Linke A, Müller P, Nurzynska D, et al.:
Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function.
Proc Natl Acad Sci U S A.
2005; 102(25): 8966–8971. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 148.
Bearzi C, Rota M, Hosoda T, et al.:
Human cardiac stem cells.
Proc Natl Acad Sci U S A.
2007; 104(35): 14068–14073. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 149.
Ellison GM, Vicinanza C, Smith AJ, et al.:
Adult c-kitpos cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair.
Cell.
2013; 154(4): 827–842. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 150.
Urbanek K, Quaini F, Tasca G, et al.:
Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy.
Proc Natl Acad Sci U S A.
2003; 100(18): 10440–10445. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 151.
Oh H, Bradfute SB, Gallardo TD, et al.:
Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction.
Proc Natl Acad Sci U S A.
2003; 100(21): 12313–12318. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 152.
Matsuura K, Nagai T, Nishigaki N, et al.:
Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes.
J Biol Chem.
2004; 279(12): 11384–11391. PubMed Abstract
| Publisher Full Text
- 153.
Takamiya M, Haider KH, Ashraf M:
Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration.
PLoS One.
2011; 6(9): e25265. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 154.
Uchida S, De Gaspari P, Kostin S, et al.:
Sca1-derived cells are a source of myocardial renewal in the murine adult heart.
Stem Cell Reports.
2013; 1(5): 397–410. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 155.
Hoch M, Fischer P, Stapel B, et al.:
Erythropoietin preserves the endothelial differentiation capacity of cardiac progenitor cells and reduces heart failure during anticancer therapies.
Cell Stem Cell.
2011; 9(2): 131–143. PubMed Abstract
| Publisher Full Text
- 156.
Liu J, Wang Y, Du W, et al.:
Sca-1-positive cardiac stem cell migration in a cardiac infarction model.
Inflammation.
2013; 36(3): 738–749. PubMed Abstract
| Publisher Full Text
- 157.
Weinberger F, Mehrkens D, Friedrich FW, et al.:
Localization of Islet-1-positive cells in the healthy and infarcted adult murine heart.
Circ Res.
2012; 110(10): 1303–10. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 158.
Di Felice V, Zummo G:
Stem cell populations in the heart and the role of Isl1 positive cells.
Eur J Histochem.
2013; 57(2): e14. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 159.
Chong JJ, Reinecke H, Iwata M, et al.:
Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart.
Stem Cells Dev.
2013; 22(13): 1932–1943. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 160.
Yaniz-Galende E, Roux M, Nadaud S, et al.:
Fibrogenic Potential of PW1/Peg3 Expressing Cardiac Stem Cells.
J Am Coll Cardiol.
2017; 70(6): 728–741. PubMed Abstract
| Publisher Full Text
- 161.
Galvez BG, Sampaolesi M, Barbuti A, et al.:
Cardiac mesoangioblasts are committed, self-renewable progenitors, associated with small vessels of juvenile mouse ventricle.
Cell Death Differ.
2008; 15(9): 1417–1428. PubMed Abstract
| Publisher Full Text
- 162.
Barbuti A, Galvez BG, Crespi A, et al.:
Mesoangioblasts from ventricular vessels can differentiate in vitro into cardiac myocytes with sinoatrial-like properties.
J Mol Cell Cardiol.
2010; 48(2): 415–423. PubMed Abstract
| Publisher Full Text
- 163.
Smart N, Bollini S, Dubé KN, et al.:
De novo cardiomyocytes from within the activated adult heart after injury.
Nature.
2011; 474(7353): 640–4. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 164.
Hierlihy AM, Seale P, Lobe CG, et al.:
The post-natal heart contains a myocardial stem cell population.
FEBS Lett.
2002; 530(1–3): 239–243. PubMed Abstract
| Publisher Full Text
- 165.
Martin CM, Meeson AP, Robertson SM, et al.:
Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart.
Dev Biol.
2004; 265(1): 262–275. PubMed Abstract
| Publisher Full Text
- 166.
Oyama T, Nagai T, Wada H, et al.:
Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo.
J Cell Biol.
2007; 176(3): 329–341. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 167.
Yellamilli A, van Berlo JH:
The Role of Cardiac Side Population Cells in Cardiac Regeneration.
Front Cell Dev Biol.
2016; 4: 102. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 168.
Messina E, De Angelis L, Frati G, et al.:
Isolation and expansion of adult cardiac stem cells from human and murine heart.
Circ Res.
2004; 95(9): 911–921. PubMed Abstract
| Publisher Full Text
- 169.
Tomita Y, Matsumura K, Wakamatsu Y, et al.:
Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart.
J Cell Biol.
2005; 170(7): 1135–1146. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 170.
Smith RR, Barile L, Cho HC, et al.:
Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens.
Circulation.
2007; 115(7): 896–908. PubMed Abstract
| Publisher Full Text
- 171.
Liu J, Wu P, Wang H, et al.:
Necroptosis Induced by Ad-HGF Activates Endogenous C-Kit+ Cardiac Stem Cells and Promotes Cardiomyocyte Proliferation and Angiogenesis in the Infarcted Aged Heart.
Cell Physiol Biochem.
2016; 40(5): 847–860. PubMed Abstract
| Publisher Full Text
- 172.
van Berlo JH, Kanisicak O, Maillet M, et al.:
c-kit+ cells minimally contribute cardiomyocytes to the heart.
Nature.
2014; 509(7500): 337–341. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 173.
Molkentin JD, Houser SR:
Are resident c-Kit+ cardiac stem cells really all that are needed to mend a broken heart?
Circ Res.
2013; 113(9): 1037–1039. PubMed Abstract
| Publisher Full Text
- 174.
Germani A, Foglio E, Capogrossi MC, et al.:
Generation of cardiac progenitor cells through epicardial to mesenchymal transition.
J Mol Med (Berl).
2015; 93(7): 735–748. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 175.
Sultana N, Zhang L, Yan J, et al.:
Resident c-kit+ cells in the heart are not cardiac stem cells.
Nat Commun.
2015; 6: 8701. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 176.
Bersell K, Arab S, Haring B, et al.:
Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury.
Cell.
2009; 138(2): 257–270. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 177.
Malliaras K, Zhang Y, Seinfeld J, et al.:
Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart.
EMBO Mol Med.
2013; 5(2): 191–209. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 178.
Mollova M, Bersell K, Walsh S, et al.:
Cardiomyocyte proliferation contributes to heart growth in young humans.
Proc Natl Acad Sci U S A.
2013; 110(4): 1446–1451. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 179.
Ali SR, Hippenmeyer S, Saadat LV, et al.:
Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice.
Proc Natl Acad Sci U S A.
2014; 111(24): 8850–8855. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 180.
Puente BN, Kimura W, Muralidhar SA, et al.:
The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response.
Cell.
2014; 157(3): 565–579. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 181.
Kimura W, Xiao F, Canseco DC, et al.:
Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart.
Nature.
2015; 523(7559): 226–230. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 182.
Leri A, Rota M, Pasqualini FS, et al.:
Origin of cardiomyocytes in the adult heart.
Circ Res.
2015; 116(1): 150–166. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 183.
Ausma J, Litjens N, Lenders MH, et al.:
Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat.
J Mol Cell Cardiol.
2001; 33(12): 2083–2094. PubMed Abstract
| Publisher Full Text
- 184.
Kubin T, Pöling J, Kostin S, et al.:
Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling.
Cell Stem Cell.
2011; 9(5): 420–432. PubMed Abstract
| Publisher Full Text
- 185.
Lyngbæk S, Schneider M, Hansen JL, et al.:
Cardiac regeneration by resident stem and progenitor cells in the adult heart.
Basic Res Cardiol.
2007; 102(2): 101–114. PubMed Abstract
| Publisher Full Text
- 186.
Kikuchi K, Poss KD:
Cardiac regenerative capacity and mechanisms.
Annu Rev Cell Dev Biol.
2012; 28: 719–741. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 187.
Lavine KJ, Epelman S, Uchida K, et al.:
Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart.
Proc Natl Acad Sci U S A.
2014; 111(45): 16029–16034. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 188.
Epelman S, Liu PP, Mann DL:
Role of innate and adaptive immune mechanisms in cardiac injury and repair.
Nat Rev Immunol.
2015; 15(2): 117–129. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 189.
Ubil E, Duan J, Pillai IC, et al.:
Mesenchymal-endothelial transition contributes to cardiac neovascularization.
Nature.
2014; 514(7524): 585–590. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 190.
He L, Huang X, Kanisicak O, et al.:
Preexisting endothelial cells mediate cardiac neovascularization after injury.
J Clin Invest.
2017; 127(8): 2968–2981. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 191.
Zhang H, Lui KO, Zhou B:
Endocardial Cell Plasticity in Cardiac Development, Diseases and Regeneration.
Circ Res.
2018; 122(5): 774–789. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 192.
Manner J:
Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium.
Anat Rec.
1999; 255(2): 212–226. PubMed Abstract
- 193.
Muñoz-Chápuli R, Macías D, González-Iriarte M, et al.:
[The epicardium and epicardial-derived cells: multiple functions in cardiac development].
Rev Esp Cardiol.
2002; 55(10): 1070–1082. PubMed Abstract
- 194.
Manner J, Schlueter J, Brand T:
Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos.
Dev Dyn.
2005; 233(4): 1454–1463. PubMed Abstract
| Publisher Full Text
- 195.
Zhou B, Honor LB, He H, et al.:
Adult mouse epicardium modulates myocardial injury by secreting paracrine factors.
J Clin Invest.
2011; 121(5): 1894–1904. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 196.
von Gise A, Pu WT:
Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease.
Circ Res.
2012; 110(12): 1628–1645. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 197.
Riley PR:
An epicardial floor plan for building and rebuilding the mammalian heart.
Curr Top Dev Biol.
2012; 100: 233–251. PubMed Abstract
| Publisher Full Text
- 198.
Smits AM, Dronkers E, Goumans MJ:
The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate.
Pharmacol Res.
2018; 127: 129–140. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 199.
Mutsaers SE, Birnie K, Lansley S, et al.:
Mesothelial cells in tissue repair and fibrosis.
Front Pharmacol.
2015; 6: 113. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 200.
Gittenberger-de Groot AC, Winter EM, Poelmann RE:
Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia.
J Cell Mol Med.
2010; 14(5): 1056–1060. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 201.
Duan J, Gherghe C, Liu D, et al.:
Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair.
EMBO J.
2012; 31(2): 429–442. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 202.
Braitsch CM, Kanisicak O, van Berlo JH, et al.:
Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease.
J Mol Cell Cardiol.
2013; 65: 108–119. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 203.
Limana F, Zacheo A, Mocini D, et al.:
Identification of myocardial and vascular precursor cells in human and mouse epicardium.
Circ Res.
2007; 101(12): 1255–1265. PubMed Abstract
| Publisher Full Text
- 204.
Bollini S, Vieira JM, Howard S, et al.:
Re-activated adult epicardial progenitor cells are a heterogeneous population molecularly distinct from their embryonic counterparts.
Stem Cells Dev.
2014; 23(15): 1719–1730. PubMed Abstract
| Publisher Full Text
- 205.
Jo JO, Kim SR, Bae MK, et al.:
Thymosin β4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1α-dependent manner.
Biochim Biophys Acta.
2010; 1803(11): 1244–1251. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 206.
Banerjee I, Moore Morris T, Evans SM, et al.:
Thymosin β4 is not required for embryonic viability or vascular development.
Circ Res.
2013; 112(3): e25–28. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 207.
Marks ED, Kumar A:
Thymosin β4: Roles in Development, Repair, and Engineering of the Cardiovascular System.
Vitam Horm.
2016; 102: 227–249. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 208.
Wagner KD, Wagner N, Bondke A, et al.:
The Wilms' tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction.
FASEB J.
2002; 16(9): 1117–1119. PubMed Abstract
| Publisher Full Text
- 209.
Bing RJ, Siegel A, Ungar I, et al.:
Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism.
Am J Med.
1954; 16(4): 504–515. PubMed Abstract
| Publisher Full Text
- 210.
Opie LH:
Heart Physiology: From Cell to Circulation. (Lippincott Williams & Wilkins, Philadelphia, USA ed. 4th Edition, 2004. Reference Source
- 211.
Opie LH:
The metabolic vicious cycle in heart failure.
Lancet.
2004; 364(9447): 1733–1734. PubMed Abstract
| Publisher Full Text
- 212.
Taegtmeyer H, Wilson CR, Razeghi P, et al.:
Metabolic energetics and genetics in the heart.
Ann N Y Acad Sci.
2005; 1047: 208–218. PubMed Abstract
| Publisher Full Text
- 213.
Heather LC, Clarke K:
Metabolism, hypoxia and the diabetic heart.
J Mol Cell Cardiol.
2011; 50(4): 598–605. PubMed Abstract
| Publisher Full Text
- 214.
van der Vusse GJ, Glatz JF, Stam HC, et al.:
Fatty acid homeostasis in the normoxic and ischemic heart.
Physiol Rev.
1992; 72(4): 881–940. PubMed Abstract
| Publisher Full Text
- 215.
Giordano FJ:
Oxygen, oxidative stress, hypoxia, and heart failure.
J Clin Invest.
2005; 115(3): 500–508. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 216.
Neely JR, Denton RM, England PJ, et al.:
The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart.
Biochem J.
1972; 128(1): 147–159. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 217.
Taegtmeyer H, Golfman L, Sharma S, et al.:
Linking gene expression to function: metabolic flexibility in the normal and diseased heart.
Ann N Y Acad Sci.
2004; 1015: 202–213. PubMed Abstract
| Publisher Full Text
- 218.
Moss AJ:
Intramyocardial oxygen tension.
Cardiovasc Res.
1968; 2(3): 314–318. PubMed Abstract
| Publisher Full Text
- 219.
Kocabas F, Mahmoud AI, Sosic D, et al.:
The hypoxic epicardial and subepicardial microenvironment.
J Cardiovasc Transl Res.
2012; 5(5): 654–665. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 220.
Semenza GL:
Hypoxia-inducible factor 1 and cardiovascular disease.
Annu Rev Physiol.
2014; 76: 39–56. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 221.
Chow DC, Wenning LA, Miller WM, et al.:
Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models.
Biophys J.
2001; 81(2): 685–696. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 222.
Parmar K, Mauch P, Vergilio JA, et al.:
Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia.
Proc Natl Acad Sci U S A.
2007; 104(13): 5431–5436. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 223.
Simsek T, Kocabas F, Zheng J, et al.:
The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche.
Cell Stem Cell.
2010; 7(3): 380–390. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 224.
Urbanek K, Cesselli D, Rota M, et al.:
Stem cell niches in the adult mouse heart.
Proc Natl Acad Sci U S A.
2006; 103(24): 9226–9231. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 225.
Morrison SJ, Spradling AC:
Stem cells and niches: mechanisms that promote stem cell maintenance throughout life.
Cell.
2008; 132(4): 598–611. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 226.
Walker MR, Patel KK, Stappenbeck TS:
The stem cell niche.
J Pathol.
2009; 217(2): 169–180. PubMed Abstract
| Publisher Full Text
- 227.
Benjamin EJ, Blaha MJ, Chiuve SE, et al.:
Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association.
Circulation.
2017; 135(10): e146–e603. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 228.
Asemu G, Papousek F, Ostadal B, et al.:
Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial KATP channel.
J Mol Cell Cardiol.
1999; 31(10): 1821–1831. PubMed Abstract
| Publisher Full Text
- 229.
Faeh D, Gutzwiller F, Bopp M, et al.:
Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland.
Circulation.
2009; 120(6): 495–501. PubMed Abstract
| Publisher Full Text
- 230.
Burtscher M:
Effects of living at higher altitudes on mortality: a narrative review.
Aging Dis.
2014; 5(4): 274–280. PubMed Abstract
| Free Full Text
| Faculty Opinions Recommendation
- 231.
Hoppeler H, Vogt M, Weibel ER, et al.:
Response of skeletal muscle mitochondria to hypoxia.
Exp Physiol.
2003; 88(1): 109–119. PubMed Abstract
| Publisher Full Text
- 232.
Gilbert-Kawai E, Coppel J, Court J, et al.:
Sublingual microcirculatory blood flow and vessel density in Sherpas at high altitude.
J Appl Physiol (1985).
2017; 122(4): 1011–1018. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 233.
Poss KD, Wilson LG, Keating MT:
Heart regeneration in zebrafish.
Science.
2002; 298(5601): 2188–2190. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 234.
Jopling C, Sleep E, Raya M, et al.:
Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation.
Nature.
2010; 464(7288): 606–609. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 235.
Jopling C, Suñé G, Faucherre A, et al.:
Hypoxia induces myocardial regeneration in zebrafish.
Circulation.
2012; 126(25): 3017–3027. PubMed Abstract
| Publisher Full Text
- 236.
Nakada Y, Canseco DC, Thet S, et al.:
Hypoxia induces heart regeneration in adult mice.
Nature.
2017; 541(7636): 222–227. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 237.
Kimura W, Nakada Y, Sadek HA:
Hypoxia-induced myocardial regeneration.
J Appl Physiol (1985).
2017; 123(6): 1676–1681. PubMed Abstract
| Publisher Full Text
| Free Full Text



Comments on this article Comments (0)