Keywords
Critical care unit, central venous catheter, length of stay, nosocomial infection, survival time, survival analysis, survival function, central line associated blood stream infection, CLABSI
Critical care unit, central venous catheter, length of stay, nosocomial infection, survival time, survival analysis, survival function, central line associated blood stream infection, CLABSI
Central line associated bloodstream infection (CLABSI) is a type of infection that affects patients who utilize central venous catheter (CVC) during their hospitalization. CVC refers to any central venous access device inserted into the internal jugular, subclavian or femoral vein that terminates in the inferior vena cava or right atrium1. CVCs are commonly used in wards and critical care units, such as the intensive care unit (ICU), and they are also referred to as central lines. In accordance with The Joint Commission (2012), CVCs are essential in health care provision as they facilitate administration of medications, intravenous fluids, and hemodialysis among other functions. CVCs are used both in the in-patient and out-patient clinical care management2.
According to The Joint Commission (2012), there are a variety of CVCs available in various sizes as well as different catheter materials, for example CVCs can be single or multi-lumen (double, triple or quadruple lumen). Another categorization by design classifies them as tunnelled catheters, non-tunnelled catheters, peripherally inserted central catheters and implantable ports2. The choice of catheter is as a result of defined need and preferences of the clinical care giver or the patient. Every catheter device carries with it some risk of infection, however, the extent of risk depends on the type of catheter used3.
Healthcare associated infection (HAI) refers to infections that occur in the course of healthcare management in any setting, more specifically, the infections acquired during hospitalization are referred to as nosocomial infections2,4. US Centers for Disease Control and Prevention (2016) observed that there was ~46% decrease in CLABSIs in hospitals across the U.S.A from year 2008 to 2013, whilst about 30,100 still occurred in critical care units and wards5. There was a declaration by the European Union that policy on HAI prevention be prioritized in 20086.
Several studies have linked CLABSIs to prolonged hospitalization and increased health management cost, as well as increased morbidity and mortality2,7–12 and 13. Morgan et al. (2010) conducted a five year study, in which the authors established that HAIs, especially CLABSIs, contributed to about one third of unexpected in-hospital mortality13. Soufir et al. (1999) conducted a prospective, matched cohort study from 1st January 1990 to 31st December 1995, in two ICUs in Paris, France and deduced that for ICU survival rates, the risk of death was significantly increased in patients with catheter related septicaemia as compared to control patients who did not develop septicaemia, with a relative risk of 2.06 (95% CI, 1.16-3.68; p-value <0.05)8. Garnacho-Montero et al. (2008) found that the median time from insertion of the catheter to the development of bacteraemia was 10 days. Further, 18 out of 66 patients died in the ICU (27.3%)10.
However, various studies face a myriad of challenges which may be attributed to difficulties in estimating the mortality resulting from blood stream infections8,14 and 15. De Angelis et al. (2010) observed that when the duration of hospitalization of patients is increased, chances of utilizing invasive catheter devices increases leading to likelihood of infection14. Umscheid et al. (2011) observed that majority of researchers have not been able to relate CLABSIs autonomously with increase in mortality due to multiple causes of patient mortality such that exclusive impact of an infection may not be explicit16. Carrico and Ramírez (2007) observed that it may not be easy to determine the patients who die “with” CLABSI compared to those who die “because of” CLABSI15.
Very few studies have been carried out on CVC utilization or its relationship with CLABSI, in particular in developing countries. Methodologies utilised in some published studies are varied, with some facing validity problems, necessitating the need for more studies. Hospitals follow a distinct culture of patient safety programmes, which among other factors affect outcomes of hospitalized patients in different ways. Globally, the risk associated with nosocomial CLABSI is dire, and even though there has been application of various strategies to reduce the impact on patients, it remains a crucial problem.
This study was intended to provide results that explain survival for patients who used CVC devices and developed CLABSI during their duration of hospitalization and compare with those who do not develop the infection in Kenya. The conceptual framework as adopted from De Angelis et al., 201014 can be viewed in Figure 1.
The first state: hospital critical care admission refers to the date when patients who utilized CVC devices during hospitalization were admitted. The second state: nosocomial infection, captures the date the nosocomial CLABSI was detected. Discharge/transfer and death, refers to the date the admitted patients exited the hospital14.
The overall aim was to determine the survival probabilities and hazard rates for patients who used CVC and establish factors affecting their survival and assess how they differ with the subset of the population that develops CLABSI. The specific objectives were as follows: i) to assess the survival probabilities for persons who utilized CVC during hospitalization; ii) to compare the survival time of patients’ group with CLABSI and the group not infected; ii) to determine whether there is an association between infection status and mortality; iii) to determine whether mortality is significantly different between the two groups and to determine factors that affect survival of patients with CLABSI.
The study was conducted at critical care units of Aga Khan University Hospital, Nairobi, Kenya namely 11-bed medical-surgical ICU, 6-bed coronary care unit, 4-bed cardiothoracic intensity care unit and 16-bed high dependency unit. The focus was on acute care admitted patients, who utilized CVC access devices between 8th December, 2012 and 31st March, 2016. The data was obtained retrospectively and included the following variables: patient’s age, date of admission and discharge, discharge status, count of devices utilized, type of CVC device used, gender, infection status, event status and survival time in days. Data were obtained from the hospital’s electronic database and infection control surveillance datasheets. Infection control team of specialized nurses were involved in data collection. A CLABSI was considered nosocomial if a recognized pathogen was isolated from one or more percutaneous blood cultures after 48hrs of vascular catheterization and was confirmed to be unrelated to an infection from a different site. Determination of the source of infection was determined by both intensivists and microbiologists through clinical evaluation.
Data were extracted and exported into MS Excel 2010 where aggregation and organization was initially carried out. Data were then exported into Statistical Package for the Social Sciences (SPSS v.24), R GUI (R-3.1.1) and Stata (SE 11.1) for further data management, cleaning and analysis. Each statistical program was utilized to run suitable analyses as per the objectives of the study as well as for attainment of desired outputs.
Both exploratory and inferential analyses were utilized. Kaplan Meier curves were used to assess the survival probabilities of exposed versus un-exposed. Log rank, Breslow, Tarone- Ware, Peto and Flemington- Harrington tests helped in testing whether the survival curves between groups differed significantly. Additional statistical tests utilized were Chi-Square test of association and test of equality of proportions, as well as Man-Whitney test.
Survival analysis is a group of statistical techniques used in analysis whereby outcome variable of interest is time taken until an event occurs. In this study the event of interest was death, which occurred among hospitalized patients who had utilized central line devices. In survival analysis time variable is referred to as survival time, in this study, the length of stay. Censoring was done when a patient did not die by the end of hospitalization, meaning he/she was either discharged alive or transferred to another facility (lost for follow-up). Censoring assumptions provided that there was independence, randomness and non-informative censoring17. In particular, right censoring was utilized.
Let T denote the random variable representing survival time where T≥0. The distribution of survival times is characterized by any of three functions: the survival function (S(t)), the probability density (f(t)) or the hazard function (h(t)). Equation (1) shows an expression of a survival function
The hazard function, h(t), presents instantaneous rate upon which events occur, provided no such previous events. The hazard function h(t) is portrayed in Equation (2), it can also be referred to as a conditional failure rate.
Hazard rate is used in providing information regarding conditional failures, in model identification and as a basis of expressing survival analysis math models.
Its cumulative function (Equation (3)) produces accumulated risk up to time t,
Kaplan Meier function enables approximation of survival probabilities by use of the product limit formula. Kaplan Meier product limit formula is given by Equation (4)
The Log Rank Test is used to compare two or more Kaplan Meier Curves. The test is approximately Chi-Square particularly for large samples with G-1 degrees of freedom (df), where G is the number of groups involved as provided in Equation (5).
Log rank is used to test the null hypothesis that there is no significant difference between two survival curves. Similarly the alternative tests to Log rank are Breslow (Wilcoxon) and the Tarone-Ware; these tests apply different weights to ith failure time.
Breslow (Wilcoxon) test weights the observed minus expected score at time ti by the number at risk ni over all the groups at time ti. In this test the weights subjected at the earlier failure times are higher as compared to later failure times17.
The test statistic is as shown in Equation (6)
Tarone-Ware test statistic applies more weight to the early failure times by weighting the observed minus expected score at time t(i) by , where ni refers to the number at risk.
Flemington-Harrington test uses the KM survival estimates for entire data to determine its weights for the ith failure time. Different choices of values for p and q can be done. Weights are computed as shown in Equation (7) whereas the test statistic is similar to Equation (6).
We consider the difference in the two proportions to be given by , which is approximately normally distributed with mean zero and
if the counts are binomially distributed with the same parameter. The null hypothesis is such that P1= P2, the common estimate for the proportion
Chi Square test of independence is used to determine whether there is a significant association between two categorical variables. Chi square test statistic is as portrayed in Equation (9)
A total of 1086 patients were included in the study; males 648 (59.7%). 363 patients experienced the event of interest (death). 47 patients developed nosocomial CLABSI during their hospitalization. Table 1 summarised the participant’s demographics.
| Variable | Values | Frequency | Proportion (%) |
|---|---|---|---|
| Event status | Censored | 723 | 66.6 |
| Event | 363 | 33.4 | |
| Gender | Male | 648 | 59.7 |
| Female | 438 | 40.3 | |
| Infection status | Yes | 47 | 4.3 |
| No | 1039 | 95.7 |
Table 2 demonstrates survival time summary statistics disaggregated by infection status. Time was measured in terms of days. The average duration of 18.19 days (standard error (s.e) 0.611) was taken by patients who did not develop a nosocomial CLABSI compared to an average of 56.79 days (s.e of 5.171) taken by those who got an infection. Median days taken by the infected group were 51 days whereas those taken by non-infected group were 12 days.
| Length of stay | |||||||
|---|---|---|---|---|---|---|---|
| Infection status | Mean | N | Std. Deviation | Std. Error of Mean | Median | Minimum | Maximum |
| No | 18.19 | 1039 | 19.701 | .611 | 12.00 | 3 | 202 |
| Yes | 56.79 | 47 | 35.450 | 5.171 | 51.00 | 6 | 149 |
| Total | 19.86 | 1086 | 22.054 | .669 | 12.00 | 3 | 202 |
Test of association between infection status and event (discharge) status depict a χ2 1=6.868, p-value=0.009, which is significant at 5% level; therefore, there is a significant statistical association between the infection status and the event (discharge) status. Test of association between gender and event (discharge) status depict a χ2 1=0.705, p-value=0.401, which is not significant at 5% level; hence there is no significant association between the gender of the patient and the discharge (event) status at 5% level (Table 3). Test of association between the gender of the patient and the infection status depict a χ21= 1.446, p-value=0.229, which is not significant at 5% level; hence there is no significant association between the patient’s gender and the infection status at 5% level (Table 3).
df, degrees of freedom.
| Test | χ2 | Df | p-value |
|---|---|---|---|
| Discharge and infection status | 6.868 | 1 | 0.009 |
| Gender of the patient and discharge status | 0.705 | 1 | 0.405 |
| Gender of the patient and infection status | 1.446 | 1 | 0.229 |
Test of equality of proportions of death between CLABSI infected and group not infected by CLABSI produced a P1=0.511 and P2=0.326 with χ21=6.0647, p-value=0.014, the 95% CI [0.02752, 0.34121]. This indicates that the proportions between the two groups are significantly different at 5% level. Hence, nosocomial CLABSI subjects a patient to a higher mortality rate as compared to a patient who does not get the infection during hospitalization.
Survival probabilities were assessed graphically as well by use of survival tables. Graphic assessment was done by use of Kaplan Meier (KM) curves for all subjects (see Figure 2), in the initial period of about 50 days after admission, survival probabilities decline at relatively more close ranges as compared to the period after 50 days. The overall mean time estimate was 70.72 days (95% CI; 60.362, 81.084) and the overall median time estimate was 44 days (95% CI; 36.49, 51.51). Further, graphical analysis by use of the KM curves was done for each group (infected vs not infected). As shown in Figure 3, the initial stages indicate higher probabilities of survival among the patients with CLABSI as compared to the group of patients with no CLABSI. However, this trend changes after about 113 days where the survival probabilities of the group not infected are higher. At about 140 days of admission, the survival probabilities of infected group decline sharply. The small sample of the patients with nosocomial CLABSI compared to the rest (with no CLABSI) may have contributed to this pattern. The median number of days estimates for the group which did not have nosocomial CLABSI were 43 days (95% CI; 34.911, 51.089) whereas for the group whose patients developed nosocomial CLABSI was 76 days (95% CI; 36.836, 115.164) (see Figure 2). On the other hand, the average number of days estimates were 76.376 days (95% CI; 63.610, 89.141) and 83.807days (95% CI; 67.382, 100.232) for non-infected and infected groups respectively.
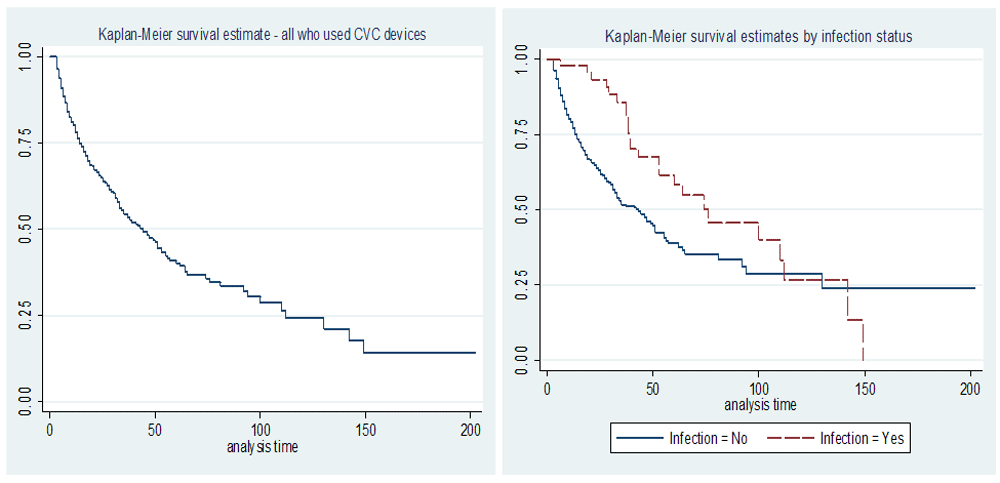
Tests on the survival curves Figure 2 were Log Rank (Mantel-Cox), Breslow (Generalized Wilcoxon), Tarone-Ware, Peto and Fleming-Harrington. Results from all the five tests show that the survival curves are significantly different at 5% level as provided in Table 4.
| Overall comparisons | |||
|---|---|---|---|
| Chi-Square | Df | Sig. | |
| Log Rank (Mantel-Cox) | 6.364 | 1 | 0.0116 |
| Breslow (Generalized Wilcoxon) | 13.954 | 1 | 0.0002 |
| Tarone-Ware | 13.326 | 1 | 0.0003 |
| Peto-peto | 11.47 | 1 | 0.0007 |
| Fleming-Harrington* | 11.23 | 1 | 0.0008 |
Test on survival time in relation to the infection status was conducted using a two sample Wilcoxon rank sum test (Mann–Whitney test) which is a distribution free/non parametric method. The value of test statistic was 6112.5 and a corresponding p-value < 2.2e-16, which indicates that it is highly significant at 5% level. We can hence deduce that there is a significant difference between the length of stay by the patients who develop nosocomial CLABSI compared to the patients who do not develop the infection. Thus the patients who develop nosocomial CLABSI stay longer in hospital.
Stratification by gender yielded the following survival curves for male and female, portrayed in Figure 3. The survival probabilities for the female subgroup were higher at the initial phase of about 40 days after admission after which the male’s survival rates remained higher until the end. The overall average duration by male patients was 73.69 days (95% CI; 60.970, 86.958), whereas that of female patients was 59.9 (95% CI, 60.970, 86.958). The average duration taken by the male patients who were infected by CLABSI was 94.324 days (95% CI; 73.6, 115.0), whereas that of female patients was 59.904 days (95% CI; 48.7, 71.1). Median was 46 days (95% CI; 34.8, 57.2) for male patients and 42 days (95% CI; 33.5, 50.5) for female patients.
Tests on survival curves by infection status after adjusting for gender using the three tests displayed on Table 5 indicate that the survival curves were all significantly different at 5% level.
| Chi-Square | Df | Sig. | |
|---|---|---|---|
| Log Rank (Mantel-Cox) | 6.499 | 1 | .011 |
| Breslow (Generalized Wilcoxon) | 13.995 | 1 | .000 |
| Tarone-Ware | 13.607 | 1 | .000 |
Tests on the survival curves with respect to gender adjusted for infection status; initially both male and female patients indicate similar survival rates from the beginning of the admission period up to about the tenth day of admission. After approximately the tenth day the female subgroup seem to portray higher survival rates up to about 45th day of admission. Stratification by patients who developed nosocomial CLABSI indicates that initially both male and female experienced similar survival rates up to approximately 6 days where the survival rates for male remain higher (see Figure 3). Three pair wise tests were conducted on survival curves with respect to gender after adjusting for infection status. Results indicate that all the three tests were not significant at 5% level.
This section portrays the survival tables for all the patients studied as well as for specific subgroups. The survival table for all the patients who were admitted into the critical care units and utilized CVCs, as provided in Table 6.
Survival table for the group who were infected by CLABSI is provided in Table 7.
The survival table for the patient group who did not get infected by CLABSI is shown in Table 8.
Assessment of the proportional hazards (PH) assumptions done using log minus log plots, observed versus expected and goodness of fit test. Assessment of the log minus log curves by infection status indicated that they were not parallel hence violating the PH assumption. Secondly, using Observed versus Expected plots; evaluated by comparing observed (KM survival estimates) versus expected (Cox adjusted) survival curve estimates plotted on the same graph with an anticipation that they would be as close to each other as possible. Results depicted a divergence between the two curves, hence violating the assumptions of PH. Lastly, using the goodness of fit test, the results depict that only the infection status doesn’t violate the PH assumption. Age, gender and number of the devices used had p-values < 0.05 indicating that violation of the PH assumption (Table 9).
| Rho | χ2 | Df | Prob>χ2 | |
|---|---|---|---|---|
| Infection status | 0.10671 | 3.74 | 1 | 0.0530 |
| Gender | 0.11674 | 5.03 | 1 | 0.0250 |
| Age | 0.14845 | 9.09 | 1 | 0.0026 |
| Count of devices | 0.16444 | 6.43 | 1 | 0.0112 |
| global test | 32.81 | 4 | 0.0000 |
Based on the results, we deduce that there is a significant difference between the lengths of stay by the patients who develop nosocomial CLABSI compared to that of patients who do not develop the infection. The patients who develop nosocomial CLABSI recorded a longer duration of stay in the hospital this in agreement with other studies conducted by14,2,7–10 and 11.
The proportion of patients who died after developing the nosocomial CLABSI compared to the proportion of those who died having not developed the infection revealed that there is a significant difference in the two proportions at 5% level (χ2 1=6.0647, p-value=0.01379). Consequently, indicating that mortality as well as morbidity is significantly increased when a patient develops the nosocomial CLABSI, similar studies by 2,7–1014 and 11 made a similar conclusion.
In the index study, the survival curves between those who developed an infection and those that didn’t were different. This difference was not due to gender or the discharge status (alive/dead) of the patient. To the best of our knowledge no such findings have been documented in similar studies.
The data having two subgroups that were considered in the study were disproportionate; the group (with the nosocomial CLABSI) had a very small proportion of 4.3% of the total number patients in the study compared to the proportion of the group that did not have the CLABSI infection during hospitalization. These disproportionate groups may not be very useful in survival model fitting. Searching for published journals from studies carried out in developing countries regarding survival analysis in relation to the use of CVC devices were not available.
The results reveal that there was a significant association between the nosocomial CLABSI infection status and the discharge status as well as the patient’s length of stay. In addition, there is a difference between the survival rates of the patients who developed nosocomial CLABSI compared to those who did not. Mortality was higher among patients who developed nosocomial CLABSI compared to those who did not during their hospitalization. The duration of hospitalization (length of stay) by the patients who developed CLABSI was significantly higher compared with the duration taken (length of stay) by patients who did not develop CLABSI, which has a great impact on the financial burden the patients are subjected to due to added hospital bed days as well as medication administered.
Since CLABSI infections have been found to elongate patient’s length of stay, appropriate strategies such as implementation of and adherence to the CVC insertion and maintenance bundles would be ideal in order to reduce the infection rates. Further matched studies in relation to CVC utilization along specific age groups and in specific diagnoses is recommended. More survival analysis research is needed in developing countries in regard to CLABSI as well as in utilization of the CVCs.
Authority to conduct the study was issued by the Ethics and Research Committee (ERC) of the participating hospital. Ethical review was conducted by the Kenyatta National Hospital/ University of Nairobi ERC, who provided approval (Ref: KNH-ERC/A/381) and an affiliation letter. Participant consent was waived since it was a retrospective study. Privacy and confidentiality was maintained by ensuring the information gathered was not relayed to anyone, but used for this study only. Patients’ names were not included in the data collected and only identification numbers utilized. No risks were subjected to the patients. Direct benefit was not intended for the study population; however the results are useful in terms of adding knowledge to the existing research.
Raw data were gathered electronically and the computer used in data analysis was personalized with limited access through password protection. No public computer was utilized in data aggregation or analysis.
F1000Research: Dataset 1. All raw data obtained in the present study. Columns A-K correspond with information contained in the sheet titled Variable_Info., https://dx.doi.org/10.5256/f1000research.16819.d22354744
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. de Grooth HJ, Timsit JF, Mermel L, Mimoz O, et al.: Validity of surrogate endpoints assessing central venous catheter-related infection: evidence from individual- and study-level analyses.Clin Microbiol Infect. 2019. PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: nosocomial infections in ICU
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Nov 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)