Keywords
Chronic Kidney Disease (CKD), ESRD, Cardiovascular Disease (CVD), Left Ventricular Hypertrophy (LVH), Left Ventricular Mass Index (LVMI), paediatric, children
Chronic Kidney Disease (CKD), ESRD, Cardiovascular Disease (CVD), Left Ventricular Hypertrophy (LVH), Left Ventricular Mass Index (LVMI), paediatric, children
Children with chronic kidney disease (CKD) are at substantially higher risk of morbidity and mortality from cardiovascular disease (CVD)1. CVD is regarded by many as the most important cause of death among patients receiving chronic renal replacement therapy; accounting for approximately 45 per cent of overall mortality2. A Task Force on CVD of the National Kidney Foundation has compared the mortality rate from CVD in dialysis patients (≈50 thousand deaths) with those in the general population (≈2 million deaths). They showed that annual mortality from CVD is much greater in dialysis patients, in spite of stratification for race, gender, or age group1. Two pathological causes of the raised risk for CVD deaths have been identified. First, the high prevalence of CVD in dialysis patients. Second, is the elevated case fatality rate among this group1.
Herzog et al. have examined the US Renal Data System between 1977 and 1995. They evaluated outcomes of more than 34 thousand patients on long-term dialysis with myocardial infarction (MI). Interestingly, they found cardiac-related deaths were ≈52 per cent at 2 years and ≈70 per cent at 5 years post-MI follow-up2.
Researchers have described three pathological forms of CVD prevalent in patients with CKD. The first one is atherosclerosis which is considered as the primary aetiology of ischemic heart disease (IHD) in that subset of patients3. Further, coronary artery plaques, in CKD patients, tend to be more advanced, with higher degrees of calcification and media thickening4. The second pathology is an alteration in heart geometry. It includes left ventricular (LV) remodelling, concentric LV hypertrophy (LVH), and eccentric LVH.
In regards to concentric LVH, the LV wall thickness rises to a greater extent than its diameter; while in the eccentric type, the magnitude of LV wall increase is in proportion to the increase in LV diameter. Concentric LVH, on the one hand, is predominantly seen in cases with pressure overload secondary to aortic stenosis, arteriosclerosis, or hypertension. On the other hand, the eccentric form can be depicted in scenarios with volume overload, such as anaemia, fluid retention, and arteriovenous fistulae5. The third pathological form is arteriosclerosis – a large vessel disease. This process involves loss of elasticity, vessel remodelling, and development of non-compliant arteries6. This results in elevated pulse pressure that, in turn, has been regarded, by many, as a risk factor for CVD deaths in dialysis patients7.
Two kinds of risk factors have been identified in CKD patients – traditional and uremia-related ones8. The traditional risk factors, that are included in the Framingham Heart Study Offspring Cohort population, include, among others, hyperlipidemia, hypertension, diabetes, male sex, LVH, and smoking. Despite the fact that CKD patients have a higher incidence of many of these risk factors, they are exposed to uremia-related ones as well8. A large number of dialysis patients have high levels of oxidative stress, homocysteine, lipoprotein(a), and lipoprotein remnants. Levels of inflammatory markers (e.g. C-reactive protein) and thrombogenic factors (such as fibrinogen) also are elevated. Furthermore, recent studies have shown that hemodialysis patients have a higher prevalence of sleep disorders, including apnea, that might contribute to the elevated risk for CVD9.
However, although the issue of CVD risks in CKD patients has been extensively studied and discussed in adults, little is known whether similar associations exist in the paediatric population. The aim of this study, therefore, is to evaluate the cardiac structure & function in children with CKD and investigate for factors that contribute to the development of CVD in that set of patients.
In this prospective cohort study, we enrolled 45 patients with CKD, treated in the nephrology unit of the Central Child Teaching Hospital-Baghdad, a high-volume, tertiary, teaching hospital over a 13-month period (from October 2016 to November 2017).
We included all the patients attending the unit during the period of the study (the sample size) with the following inclusion criteria in mind: age between 2–14 years, GFR<60 ml/min/1.73 m2 for more than 3 months, and absence of confounders, such as myocardial disease (congenital, structural or primary), diabetes mellitus (DM) or corticosteroid use during the period of study. As such, five patients were excluded as two had congenital heart diseases, another two had DM, and one was receiving corticosteroid treatment.
For comparison, we selected a control group of 40 patients (1:1 ratio) who were age- and gender-matched to the study group. Age matching was considered present if the difference was within two years. The controls were patients in the same hospital but with unrelated conditions or diseases. They have been recruited randomly from different units of the hospital. Informed written consent was taken and the following inclusion criteria were considered: normal systemic blood pressure (BP), renal function tests, general urine exam (GUE), and abdominal ultrasound (US) of the genitourinary tract with no clinical evidence of cardiovascular system issues (such as cyanosis, shortness of breath, chest pain, palpitation, and cardiac murmur).
As per this study, CKD is defined as renal damage or glomerular filtration rate (GFR) of less than 60 ml/minute/1.73 m2 body surface area (BSA) for three months, regardless of the cause10.
For all the patients (cases and controls) included in this study, we measured the following parameters: body weight (BW), height (Ht), height percentile, body mass index (BMI) (BW/Ht2), and surface area (SA). The latter is calculated as follows: SA = Square Root [BW (kg) x Ht (cm) / 3600].
The patients' medical records were reviewed for the presence of primary renal disease, its duration, underlying cause, and the medications used. In regards to the cause, we categorized the patients into four groups. Group I: congenital renal anomalies, group II: acquired renal diseases, group III: hereditary nephropathies and group IV: CKD of unknown aetiology. We recorded the mode of treatment into one of three modalities: hemodialysis (HD), continuous ambulatory peritoneal dialysis (CAPD) or conservative treatment. The outcome of the patients, during the period of the study, have been identified.
On the day of the echocardiographic study, we took the following blood samples for each patient: haemoglobin, blood urea, serum creatinine, serum calcium and serum phosphorus. All these tests were analyzed using the standard, local laboratory methods.
The estimated GFR (eGFR) has been calculated using Schwartz Formula11 (eGFR= Height (cm)/serum creatinine (mg/dl) * K). Where K is a “constant” based on age as follows: 0.70 in adolescent boys; 0.55 in adolescent females & children; 0.45 in full term infants and 0.33 in preterm newborns.
According to the eGFR, we divided the patients into two groups: those < 15 ml/min/1.73 m2 and those ≥ 15 ml/min/1.73 m2. Furthermore, we divided the duration of the CKD into two groups–either within one year or those more than or equal to one year.
Blood pressure was measured three times during the same day; in sitting position and using mercurial sphygmomanometer with an appropriate cuff size12. We took the average of the three measurements and compare the results to nomograms. Hypertension is said to be present when the averaged systolic blood pressure (SBP) and or diastolic blood pressure (DBP) equal to or exceeded 95th percentile for sex, age & height12.
Anaemia is defined as a haemoglobin level of less than 11 g/dl in prepubertal children with CKD11.
Intact parathyroid hormone (iPTH) was assessed using a Cobas e 411 Analyzer (Roche Diagnostics) at a local private lab. Hyperparathyroidism is said to be present when the iPTH exceeds 65 pg/ml13.
Two board-certified, pediatric cardiologists performed echocardiographic assessments using a Philips CX50 Ultrasound Machine (Philips Healthcare, USA) with the S5-1 transducer. They examined for valvular calcification using 2D Mode; while M-Mode has been used to assess the following: Interventricular Septum thickness in diastole (IVSd), Left Ventricular Internal Diameter in diastole (LVIDd), Left Ventricular Internal Diameter in systole (LVIDs) and Left Ventricular Posterior Wall in diastole (LVPWd). A built-in software tool was used to calculate the fractional shortening (FS %) [(LVIDd – LVIDs) / LVIDd * 100]. Left Ventricular Mass (LVM) was calculated using a computerized Microsoft Access 2016 model using Devereux & Reichek cube formula14 : LV Mass = 0.8 x 1.04 x [(LVIDd + LVPWd + IVSd)3 – LVIDd3] + 0.6. The calculated LVM is then indexed to body surface area (LVMI).
For this study an FS of 25–43% was considered to be a normal value. However, for the IVSd, LVIDd, LVIDs, and LVPWd we depend on values indexed to body surface area accordingto Park 200815.
Left ventricular hypertrophy (LVH) is defined as LVMI greater than the 95th percentile for gender and age16.
Statistical analysis was performed with IBM SPSS Statistics 23.0. Suitable graphs & tables were used to describe the data. Student's T-test was used for comparison between continuous variables. Chi-square & Fisher's exact tests were used to examine qualitative and frequency data and to find the relation between risk factors and different outcomes. P value < 0.05 was considered significant.
The cohort consisted of 80 patients divided into two groups: 40 children with CKD (cases group) and 40 children without CKD (controls group) (Datasets 1 & 2).
Some of the general characteristics of the study population are shown in Table 1.
Among the study patients, 72.5% were normotensive. However, all the control group were normotensive and 55% of cases group were hypertensive with a significant association between CKD & high blood pressure (P = 0.001) (Figure 1)
About 40% of the study population, 7.5% in the control group and 72.5% of the cases group were anaemic (Figure 2). There is a significant association between CKD and anaemia (P = 0.001).
With regards to the biochemical & hormonal studies, blood urea, serum (S.) creatinine, S. phosphorus (Po), Calcium * Phosphorus (Ca * Po) product and parathyroid hormone (PTH) levels were higher amongst patients in the case group than in the controls. However, S. Ca is lower in the cases (Table 2).
Most of the patients in the cases group had CKD for more than a year (62.5%), and the majority (82.5%) were alive during the period of the study (Figure 3 & Figure 4). More than half of them were treated conservatively, however, other modalities were utilized (Figure 5).
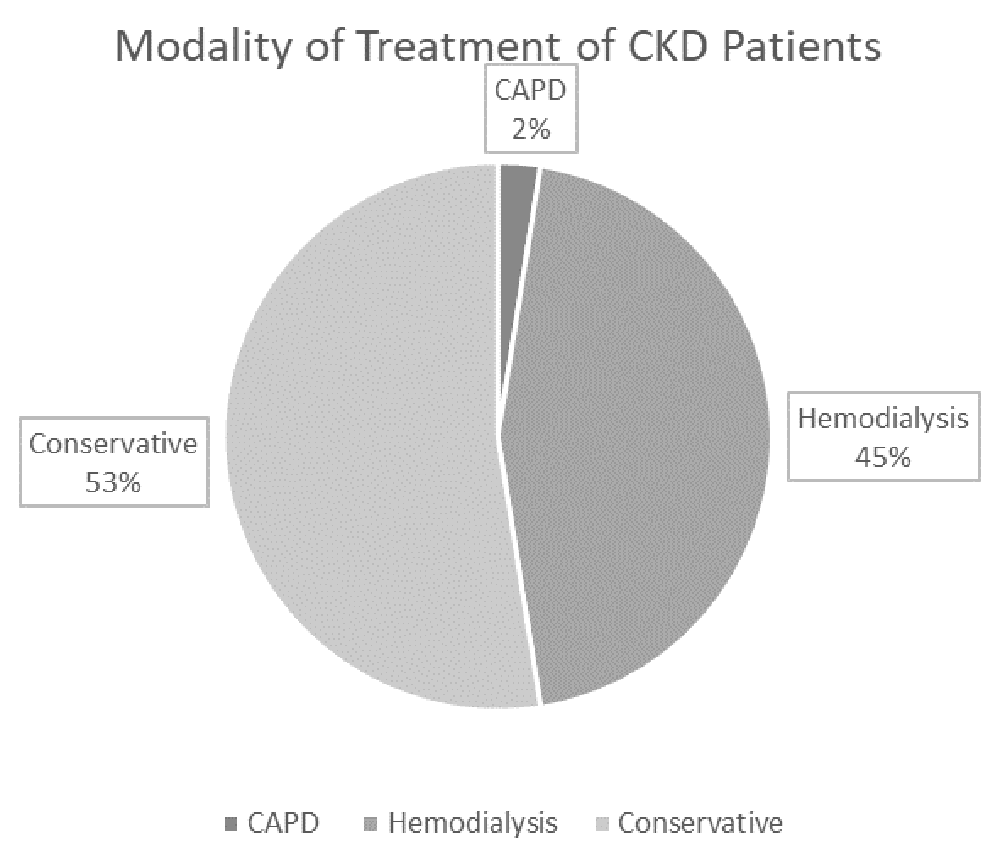
CAPD – continuous ambulatory peritoneal dialysis.
Congenital renal abnormalities constitute 37.5% of the causes of CKD in this study, with vesicoureteral reflux (VUR) being the most common anomaly seen (32.5%) (Table 3).
CKD Patients were found to have a higher left ventricular mass index (LVMI) when compared to the control group (75.5% vs. 0%) with a statistically significant association between CKD and LVH (P = 0.001). However, we did not find any significant association between CKD and both valvular calcification, and fractional shortening (FS) (P = 0.314) (Table 4).
In terms of the cardiac measurements, interventricular septal diameter in diastole (IVSd), left ventricular posterior wall diameter in diastole (LVPWd), and LVMI are higher (P < 0.05) among CKD patients than the controls. On the other hand, there is no statistical significant difference (P > 0.05) in term of FS, LV internal diameter in diastole (LVIDd) and LV internal diameter in systole (LVIDs) between the CKD patients and the controls (Table 5).
Abbreviations: CI – Confidence Interval; FS – Fractional Shortening; IVSd – Interventricular Septal thickness in Diastole; LVIDd – Left Ventricular Internal Diameter in Diastole; LVIDs – Left Ventricular Internal Diameter in Systole; LVMI – Left Ventricular Mass Index; LVPWd – Left Ventricular Posterior Wall in Diastole
High LVMI was seen in patients with all of the following: anaemia, hypertension, CKD duration > one year, high PTH and eGFR < 15 ml/min/1.73 m2. All the patients who died, as well as those with acquired or unknown cause of CKD, have increased LVMI. On the other hand, no association has been discovered between gender or mode of treatment, and the risk of high LVMI (Table 6).
In this study, 19 patients (47.5%) – in the cases group – were males and 21 cases (52.5%) were females; with a male: female ratio of 0.9: 1. However, this contradicts results obtained in other studies, like Harambat et al.17 where the higher prevalence of CKD in males has been attributed to the higher frequency of congenital abnormalities of the kidney and urinary tract (CAKUT) in this group.
Body mass index (BMI) does not reflect body composition. Furthermore, a patient who has an appropriate BMI for age might not have a typical body composition17. In this study, BMI shows no significant difference (P = 0.812) between the cases and the controls groups. This is consistent with previous studies18,19
In terms of blood pressure, 55% of patients in the cases group were hypertensive (P = 0.001), though other studies show a higher prevalence20. Many factors contribute to the raised incidence of hypertension in CKD patients. They include sodium retention, increased activity of the renin-angiotensin-aldosterone system, an exaggerated activity of the sympathetic nervous system, secondary hyperparathyroidism, deficient nitric oxide and endothelium-mediated vasodilation, and treatment with erythropoietin – if present20–24. In addition, hypertension might be a causative (e.g. hypertensive nephrosclerosis) or contributary pathology in the development of CKD25.
Anaemia was found in 29 patients (72.5%) in the cases group (P = 0.001). This is consistent with results obtained by other researchers26–29. A hypoproliferative state is thought to be the underlying mechanism. The latter might result from the following: First, changes in iron metabolism which is hepcidin-induced that causes decreased iron absorption from the gut, in addition to iron macrophage trapping30,31. Second, shortened red blood cells (RBC) survival, resulting from raised macrophage activity32. Third, a high apoptotic death of RBC precursors in the bone marrow33. Finally, a "relative" decline in erythropoietin (EPO) production31.
In the United States and Europe, many studies have shown that congenital genitourinary anomalies account for almost 60% of CKD cases in younger children; while glomerular diseases are more common in older children & adolescents34–36. In the first subset of patients, obstructive uropathy (21%) is the most common pathology, followed by renal dysplasia/hypoplasia (18%) and reflux nephropathy (8%). On the other hand, focal segmental glomerulosclerosis (FSGS) is the most prevalent glomerular lesion, accounting for 9% of cases. However, in our study, congenital renal anomalies were found in 15 patients (37.5%) with vesicoureteral reflux (VUR) being the most common pathology (N = 13; 32.5%). Both FSGS and rapidly progressive glomerular nephritis (RPGN) have the same prevalence as a cause of acquired glomerular diseases (N = 2; 5%).
Left ventricular hypertrophy (LVH) is regarded by many as an adaptive response to long-lasting volume or pressure overload. Initially, it is beneficial because it allows for increasing work capacity, maintains systolic function and decreases energy consumption & wall stress. However, with time, LVH becomes detrimental as it alters LV diastolic function, decreases coronary perfusion reserve and predisposes to arrhythmias & sudden death37–39. Some researchers40,41 have found that an increase in LV mass by 1 gm/m2.7/month is associated with a 62% rise in the risk of fatal and non-fatal cardiovascular events in dialysis patients. In our study, all the seven children who died had LVH.
In this study, CKD patients had a thicker diastolic interventricular septum (IVSd) (10.5 vs 6.4, P = 0.001), diastolic LV posterior wall (LVPWd) (10.7 vs 6.2, P = 0.001) and LV Mass Index (LVMI) (150.1 vs 54.7, P = 0.001) in comparison to controls. Furthermore, LVMI was increased in 27 (75.5%) patients in the cases group (P = 0.001). These results are consistent with those obtained by Malikenas et al.42 and van Huis et al.43.
Despite the fact that LV systolic dysfunction carries a poor prognosis in end-stage renal disease (ESRD) in adults, and is considered by many as an important co-morbidity, LV function is usually well preserved in the pediatric population43. In our study, only one case (2.5%) shows low LV fractional shortening with no statistically significant association between CKD & systolic dysfunction. This is consistent with other researchers in other parts of the world43,44. It may be that LV systolic dysfunction only becomes clinically evident after a long period of time.
Many studies have shown that vascular & valvular calcification is associated with cardiovascular disease, which is by far the most common cause of death in CKD45,46. Calcification is prevalent among CKD patients, particularly those on dialysis. Prevalence is highest among patients with lower eGFR, and it is presumed that, as the eGFR declines, the prevalence as well as the severity of the calcification rise47–49. It has been seen that 80% of dialysis patients show calcification versus 47–83% among those who are not on dialysis48–56.
In our study however, valvular calcification was present in only one patient (2.5%). This low prevalence can be explained as follows: first, all the studied patients are under the age of 14 years. Knowing that calcification is a slowly-developing process that takes time, the younger the patient, the less likely this pathology can be clinically detected. This was shown in a comprehensive systematic meta-analysis review of 30 studies over a 20-year-period that demonstrated that the age of the patient is one of the most important factors associated with calcification in CKD50. Second, we relied on echocardiography in detection and quantification of valvular calcification. However, computed tomography (CT), using special software & scanners is considered, by many, as a more sensitive tool in this context, especially when coronary arteries calcification needs to be assessed as well57,58. Third, preventive measures, in our centre, may play a role, which includes the cautious use of vitamin D therapy and achieving net calcium balance by using non-calcium-containing phosphate binders or by modifying the dialysate bath calcium concentration in dialysis patients59,60.
In adults, many studies have shown an association between LVH and parathyroid hormone (PTH) concentration in patients with CKD61,62. The underlying mechanisms of PTH-induced LVH include an indirect effect – by elevating systemic blood pressure, and a direct effect of this hormone on the cardiac myocytes62. Furthermore, in vitro studies have revealed that PTH has inotropic, chronotropic as well as hypertrophic effects on cardiac cells63,64. In this study, high LVMI was present in 26 out of 30 cases (86.7%) with high PTH. The latter is consistent with results obtained by Mitsnefes et al.65 who showed that raised PTH level is associated with LVH progression in CKD stages 2–4.
In our study, LVH was found in 24 out of 29 cases (82.8%) with anaemia. This is consistent with most studies in children, which revealed a significant relationship between anaemia and raised LVMI65–68. However, recent studies in adults with mild-moderate CKD or on long-term dialysis showed that increasing haemoglobin (Hb) levels was not associated with LVH regression69–72. Therefore, the authors have suggested that the relationship between LVH and anaemia could not be causal. Of note, these studies recruited subjects with relatively mild anaemia and, thus, not able to answer the question of whether correcting more severe anaemia may lead to a decline of LVMI. On the contrary, Morris et al.73 observed a substantial reduction in LVMI with the treatment of severe anaemia in children on dialysis.
Regarding the mode of treatment of CKD and its association with LVH, research74,75 has shown that the prevalence of LVH varies with the mode of treatment of CKD; being 16–31 % in those with eGFR > 30 ml/min/1.73 m2, 60–75% when renal replacement therapy is started, and 70–90% in patients with regular dialysis. Our study, however, reveals rather different statistics. Up to 50% of patients on conservative treatment had LVH. Refusal to start renal replacement therapy by the family has caused a substantial delay in the treatment and this might be the most likely explanation of these figures.
The lower the eGFR, the more likelihood of having LVH. In this study, more than 85% of patients with eGFR of < 15 ml/min/1.73 m2, in comparison to 47% of those whom eGFR ≥ 15 ml/min/1.73 m2. These findings are consistent with those of researchers elsewhere in the world44,74,75.
Malikenas et al.42 have noticed an association between the CKD causes and LVH. They found that patients with acquired renal diseases had significantly higher LVMI than those with congenital renal abnormalities. In our study, 100% of patients with acquired causes had high LVMI versus 53% with congenital anomalies. An interesting point, however, is that 100% of those with unknown cause of CKD had LVH. This can be explained by the fact that these cases are usually presented late to our centre.
1. Because of time constraints, only one assessment has been done to both the cases and the controls groups. We believe that with frequent, lengthy follow up on the cases arm, many parameters would change with time, such as LV mass & function, calcification, outcome, etc. Thus, an extension of this study is in progress to include months of follow-up.
2. To detect cardiovascular calcification, we depended on echocardiographic detection. However, computed tomography (CT) is considered, by many, as a more sensitive tool in this context.
3. Only left ventricular (LV) systolic function has been assessed, using LV fractional shortening (FS). While neither LV diastolic, nor right ventricular (RV) function has been evaluated, though we think both might be influenced in patients with CKD.
Despite the fact that LV systolic function is usually well-preserved & valvular calcification is usually absent, LVH is common in children with CKD. LVH is associated with higher mortality in that subset of patients; with the severity & duration of renal impairment, hypertension, anaemia, and hyperparathyroidism are amongst the additional risk factors that predispose to LVH. We contribute this study to the growing information of the review articles regarding the association between CKD and cardiovascular complications in paediatrics. However, additional clinical trials are urgently required with a goal to reduce CVD in CKD children.
All the raw data required for reproducibility of this study are available on Open Scientific Framework
OSF. Dataset 1 & 2: Replication Data for: Toward Cardiovascular Risks in Children with Chronic Kidney Disease, https://doi.org/10.17605/OSF.IO/WY28B76
Data is available under a CC0 1.0 Universal license
The Institutional Ethics Committee of the Paediatric Department, College of Medicine, Al-Mustansiriyah University has reviewed & discussed the initial application to conduct this study, informed about its progress, and any revision in the protocol and patient information/informed consent. The Committee has received & approved the final report of the study on April, 2018.
None of the investigators participating in this study took part in the decision making & voting for this study.
Informed written consent was taken from the parents of the children to participate in this study and for publication of the clinical details.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glomerular disease, workforce, communication
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chronic kidney disease, cardiovascular disease in chronic kidney disease, community acquired acute kidney injury.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Nov 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)