Keywords
Enterococcus, biofilm, endodontics, Zingiber officinale
Enterococcus, biofilm, endodontics, Zingiber officinale
Enterococcus faecalis is an opportunistic facultative anaerobe that is well recognised as an oral pathogen associated with persistent apical periodontitis and is highly prevalent in failed root filled teeth1. The ability of E. faecalis to enter and survive in root canals, as well as its resistance to root canal medicaments, makes it one of the toughest pathogens to eradicate in endodontics2–7. It has been reported that this pathogen is associated with a high percentage of endodontic failures, which is about one third of the canals of root-filled teeth with persistent periapical lesions8. Inevitably, repeated use of calcium hydroxide and sodium hypochlorite as the two most commonly used root canal medicaments and irrigation solution, respectively, has been said to allow E. faecalis to adapt to the sub-lethal environment9.
Within the past decade, herbal medicine has begun to offer some beneficial antibacterial activities against oral pathogens10,11. More recently, studies also found the potential antibacterial properties of ginger on oral pathogens, including E. faecalis12–16. In most instances, the effects of ginger extracts were studied on bacteria cultured in vitro and in cell suspensions, using calcium hydroxide or sodium hypochlorite as a control. Limited data is available on the activity of ginger oil in disrupting established E. faecalis biofilm and its comparable effect to antibiotics. Hence, the aim of our study was to explore the in vitro potential of ginger oil as an antibacterial agent against E. faecalis cultured in suspension and biofilm in comparison to ampicillin, as a common antibiotic used for oral infection.
Ginger oil was prepared from Zingiber officinale Roscoe rhizomes after fresh young ginger were finely sliced, dried and boiled in distilled water for 8h17. The oil stock solution (500 mg/mL) was prepared by mixing 100 µL ginger oil with 200 µL dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO USA) in an Eppendorf tube. Next, 100 µL of the oil stock solution was pipetted and mixed with 100 µL DMSO in a second tube to produce two-fold dilution at 250 mg/mL. This procedure was repeated six times to produce an eight concentration series of ginger oil solution at 500, 250, 125, 62.5, 31.3, 15.6, 7.8, 3.9 mg/mL respectively. The solutions were vortexed each time after mixing to ensure a thorough mixture were produced. Following this, 20 µL of each oil series were transferred into a 980 µL brain heart infusion (BHI; Oxoid Ltd., Cheshire UK) broth in 2.5 mL universal bottles to produce eight working concentration series of ginger oil mixtures in broth microdilution test at 10, 5, 2.5, 1.25, 0.63, 0.31, 0.16 and 0.08 mg/mL respectively. The positive control ampicillin solution was prepared by mixing 500 mg ampicillin powder (Sigma-Aldrich) with 1 mL distilled water, and then eight series of two-fold solutions were prepared similarly to the technique mentioned above.
Enterococcus faecalis ATCC29212 (American Type Culture Collection, Virginia USA) used in this study was cultured in BHI (Oxoid Ltd., Cheshire UK) agar plates and regularly maintained in a 37°C incubator with 5% CO2. Identification and purification of E. faecalis were done through Gram stain test as well as morphology and colony identification. The quantity and viable bacterial number of E. faecalis were determined by colony forming unit (CFU) and standardized with McFarland 0.5 turbidity (1.5 x 108 cfu/mL) prior to the antibacterial assays. Cultures and broths were constantly checked for sterility and contamination. DMSO was used as solvent for ginger oil and ampicillin as the positive control.
Research and ethics approval was obtained from the Universiti Kebangsaan Malaysia Research and Ethics Committee (UKM 1.5.3.5/244/DD/2014/017) for methodology and dissemination of findings.
An antibacterial assay using the broth microdilution technique was done based on modified NCCLS guideline18. A 96-well test plate was divided into two sections where half was inoculated with 50 µL E. faecalis suspension in each well and another section was not inoculated (50 µL broth/well only), as shown in Figure 1, plate rows from A to H. Sample solution series prepared earlier were mixed with the bacterial suspensions in wells and produce a final concentration series of oil mixtures at final concentrations of 5, 2.5, 1.25, 0.63, 0.31, 0.16, 0.08 and 0.04 mg/mL respectively19. One hundred microliters sample series (ginger oil mixture or ampicillin solution) were dispensed into the prepared 96-well plate. Triplicates were done for each well and test plate. Plates were then incubated at 37°C for 24h. After this, the turbidity of wells on tested plates was measured at 595nm optical density using a microtiter plate reader (Thermo Scientific VARIOSKAN Flash, UK). Bacterial growth inhibition percentage was calculated using the following formula:

The reading generated by the device were analysed and the Minimum Inhibitory Concentration (MIC), defined as the lowest concentration of sample that inhibits visible growth of a microorganism after overnight incubation, was determined. Further to this, 20 µL of the mixture from each well that showed bacterial growth inhibition were cultured on BHI agar plates for 24h at 37°C in order to determine whether the inhibition is bacteriostatic or bactericidal in nature. The minimum bactericidal concentration (MBC), defined as the lowest concentration of samples that prevent the total growth of E. faecalis on test plates20, was determined.
A monospecies biofilm was initially produced by inoculating 200 µL E. faecalis suspension culture at 1.5 x 108 cfu/mL cell density with BHI broth in 96-well plates (modified from Azizan et al19). The culture was checked daily for contamination for 4 days and cell density was measured at 595 nm optical density using a microtiter plate reader, and repeated in three separate tests. The findings from this monospecies biofilm development test was charted (Figure 2) and used to determine the age for a stable pre-formed biofilm for the anti-biofilm assay.
In the anti-biofilm assay, E. faecalis biofilm was developed and maintained for 3 days. On the third day, culture broth in the plate was pipetted out in slanting position to prevent disruption of biofilm and replaced with 100 µL of fresh broth. Then 100 µL ginger oil or ampicillin solutions at eight respective concentrations were dispensed into the wells and plates were incubated at 37°C for 24h. The turbidity of the bacterial cultures was measured using a microtiter plate reader at 595 nm optical density.
Each assay was conducted in triplicate and two independent experiments were done. The data collected were analysed with SPSS 21.0. Non-parametric Mann-Whitney test were used with significant level set as 0.05. Results were accepted as statistically significant if the p value was <0.05.
The broth microdilution antibacterial assay showed a dose-dependent inhibition of E. faecalis growth when exposed to all tested concentration series of ginger oil solution (Figure 3), but no bactericidal effect of the oil was found within the range tested (0.04 – 5.0 mg/mL). While ampicillin showed almost 80% inhibition for all concentrations tested, ginger oil showed about 76% inhibition at 5.0 mg/mL and 50% and less at 2.50 – 0.04 mg/mL. The difference in means of inhibition between all ginger oil concentrations tested and between ginger oil and ampicillin was found to be statistically significant (p=0.002 and p=0.007, respectively).
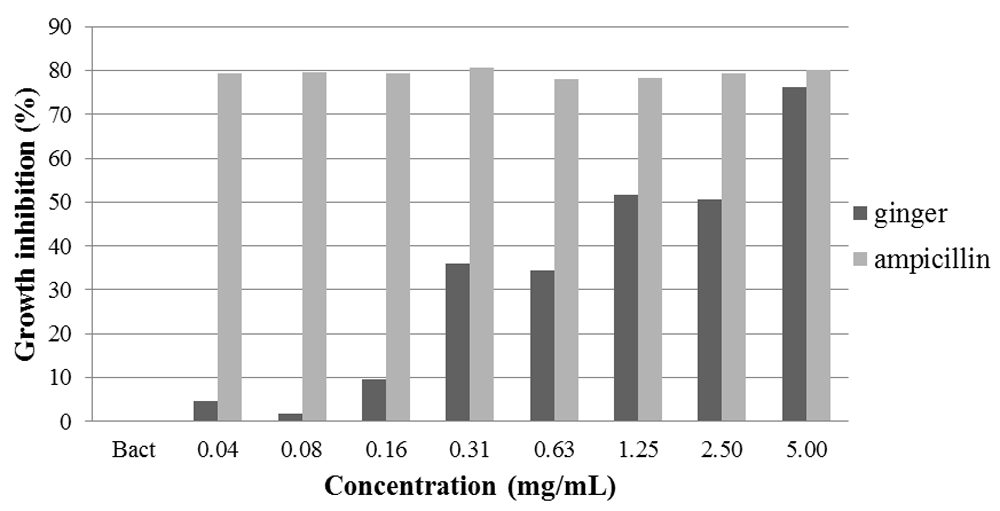
* significant difference (p<0.05) between ginger oil and ampicillin at 5.00mg/mL.
In the anti-biofilm test, ginger oil also inhibited the growth of pre-formed biofilm (Figure 4). However, it was found that both ginger oil and ampicillin showed lesser inhibitory effect on pre-formed biofilm than on suspended cells in broth (Figure 3). In this test, the anti-biofilm activity of ginger oil was observed as an increase between 1.25 to 5 mg/mL, yet the activity failed to achieve an acceptable level of inhibition of more than 50%. At 1.25 mg/mL, ginger oil was seen to have somewhat equal biofilm disruptive property as ampicillin, but the means of anti-biofilm activity between the two agents showed no statistically significant difference (p=0.052).
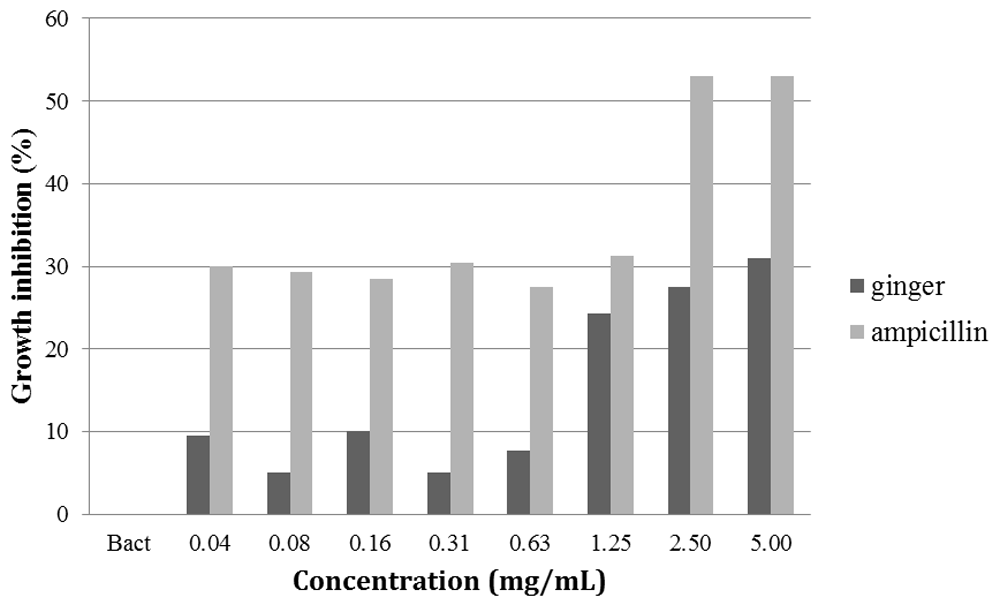
It has been advocated that ginger, as the stem of Z. officinale plant, has strong antimicrobial properties including antibacterial and antifungal against pathogens, including bacteria from the oral cavity21–27. In the present study, it was further discovered that ginger rhizome oil exhibited bacteriostatic activity on E. faecalis cultured in plates as monospecies suspension and causes disruption of the pre-formed biofilm. The dose-dependent effect of the oil was accepted as a positive outcome of the study and we predict that with higher concentration (more than 5 mg/mL), ginger oil would have bactericidal effect on E. faecalis.
The ability of ginger oil to disrupt 3-day old pre-formed monospecies E. faecalis biofilm provided us with a new insight on the antibacterial property of this herbal oil. Although the activity was not as potent as the effect on suspension culture, ginger oil still produced acceptable inhibitory activities against the pathogen in vitro. This study provides clearer appreciation of the E. faecalis resistance to antibacterial agents when they are in biofilm form and conforms to other evidence of biofilm resistance28–30.
The use of systemic antibiotics such as ampicillin in acute endodontic infections has raised many concerns over the increase in the prevalence of bacterial resistance in dentistry31–33. On the other hand, the current use of antibacterial irrigation solutions such as sodium hypochlorite, chlorhexidine and iodine as well as calcium hydroxide as canal medicament reduces the needs for systemic antibiotics. However, some degree of toxicity towards vital tissues and allergy reactions to some patients have been reported34–36. Through our study, we found that ginger oil has lower but comparable antibacterial efficacy against E. faecalis compared to ampicillin. Further studies to investigate the effects of combined oil-antibiotics may perhaps provide some useful information on the range of its antibacterial activities. Studies have shown that combination of herbal oils with antibiotics does produce adequate synergistic and additive effects against microorganisms37.
Lastly, the results of this study demonstrated that at determined concentrations, ginger oil has the potential to be used as an antibacterial agent in the management of failed root canal therapy. Our recent study on the effect of ginger oil on tooth dentine microhardness also provide promising usage of this herbal oil in future endodontics as it offers comparable changes to dentine structure when used as irrigation solution in contrast to sodium hypochlorite and ethylenediaminetetraacetic acid38. These two findings may help to access infection deep within the root canal system. Nevertheless, the current clinical scenario does not offer immediate and accurate chairside information on the type of bacterial species involved in specific endodontic infection; hence this finding may not be readily useful for purpose as yet. More studies will be required to evaluate the clinical efficacy of the delivery of ginger oil as an antibacterial agent for endodontic infections.
Within the concentration tested (0.04 – 5.0 mg/mL), ginger oil showed an acceptable dose-dependent in vitro inhibitory activity against E. faecalis (MIC 0.04mg/mL), while its antibacterial activity towards bacteria in suspension broth was comparatively better than its anti-biofilm activity.
F1000Research: Dataset 1. File containing raw data for biofilm development test, anti-biofilm assay and suspension assay. , https://doi.org/10.5256/f1000research.16851.d22512239
This study was funded by the Universiti Kebangsaan Malaysia Young Researchers’ Incentive Grant (GGPM-2013-096).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors wished to thank Miss Nurulnuha Kamal Baharin of Faculty of Dentistry UKM Microbiology Lab, for her assistance in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral environment, Oral biofilm, Antimicrobial agent
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. EUCAST: EUCAST Quality Control. [accessed 15th Jan 2019]. Reference SourceCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: infectious diseases, clinical microbiology, antibiotic resistant bacteria, biofilms, enterococci,
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Nov 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)