Keywords
renovascular disease, renal angioplasty, microcirculation, angiogenesis, inflammation, mitochondria
renovascular disease, renal angioplasty, microcirculation, angiogenesis, inflammation, mitochondria
Renal artery stenosis is the main cause of chronic renovascular disease (RVD), which in over 90% of cases is due to obstructive atherosclerosis of the main renal artery or primary bifurcations. The buildup of atherosclerotic plaques in the renal arteries is a late reflection of a systemic process that also affects other vascular territories. Indeed, the presence of atherosclerotic RVD (ARVD) may reflect widespread vascular disease, including atherosclerotic disease of the coronary arteries, cerebral blood vessels, and peripheral blood vessels, which together account for the high prevalence of cardiovascular morbidity and vascular-related deaths of patients with ARVD. The widespread nature of atherosclerosis may also be a major contributor to the difficulties in defining a comprehensive therapeutic approach for patients with ARVD. Furthermore, other factors make it challenging to develop a “one size fits all” therapy for ARVD, such as uncertainty regarding the duration of the disease and the likelihood of coexistent comorbidities like pre-existing hypertension, obesity, diabetes, or smoking.
Randomized controlled trials (for example, STAR, DRASTIC, ASTRAL, and CORAL), several smaller studies, and case reports support the notion that interventional strategies that can fully resolve renal artery stenosis may not offer an advantage over medical therapy alone1–4. These comparative studies show no significant differences between percutaneous transluminal renal angioplasty and stenting (PTRAS) versus medical therapy in outcomes of hypertension, renal function, or cardiovascular risk, consequently failing to justify the inherent risks of a direct intervention (catheter-based with and without stenting or surgical revascularization) on the obstructed renal artery to treat ARVD. Nevertheless, patients who improve their renal function after PTRAS have significantly better survival rates compared with those who do not improve5. Therefore, it might be reasonable to take a step back (or ahead) in ARVD and place current efforts on not only identifying those patients who are most likely to benefit from one strategy or the other and why, but also better understanding the pathophysiology of ARVD. This approach will permit evaluation of the potential for combined strategies that not only address renal artery stenosis, but also protect the renal parenchyma distal to the vascular obstruction.
ARVD is a complex disease. An inherent challenge in the diagnosis of ARVD (via ultrasound of the renal arteries, magnetic resonance angiography, or contrast-enhanced angiogram)6 is the frequent inaccuracy of the angiographic determination of the severity of the stenosis, which may contribute to a delayed diagnosis and missed opportunities for interventions with predictable outcomes4. Another challenge is that no specific clinical characteristic in patients with ARVD can reliably predict the renal functional outcome after treatment7. Furthermore, for several years, all the attention was on resolving the renal vascular obstruction. It is undeniable that a severe, hemodynamically significant stenosis (defined as a reduction of at least 70% of the arterial lumen or at least 50% of the area) is a prominent insult for the development of renal ischemia and hypertension, as has been shown in elegant studies using translational animal models of ARVD as well as in the clinical setting. Nevertheless, the results of the two largest randomized controlled trials—ASTRAL and CORAL—that performed a comparative evaluation of the therapeutic efficacy of PTRAS versus full medical therapy resulted in the boldest testimony against catheter-based strategies for ARVD1,2,8: there seems to be no significant advantage of PTRAS over medical therapy.
However, open questions remain. Recent scrutiny of these studies highlighted important flaws in the design that may have played a role in some of the clear but often misleading results and subsequent conclusions. In-depth discussion of their design strengths and weaknesses is beyond the scope of this article, and the reader may consult recently published literature4,9. A major criticism of these trials includes the fact that almost 50% of the recruited patients showed renal artery stenosis of less than 70% and normal renal function or subclinical (stage I or II) renal dysfunction3. Thus, clinical characteristics (underlying pathophysiology) of almost half of the patients may have played a role in the indifferent results of the trials. Furthermore, evidence obtained by subanalysis of these trials supports the notion that renal artery stenosis of at least 80% (associated or not with severe uncontrolled hypertension), patients with a rapid deterioration of renal function, and patients presenting with flash pulmonary edema distinctly benefited from PTRAS3,10,11. The selective but limited beneficial effects of PTRAS for ARVD highlight the complexity of the disease and suggest that factors prior to and beyond the vascular obstruction may likely be determinants in the outcome. These results also emphasize that vascular obstruction is an important component but may not be the only abnormality that should be treated. Finally, the provocative findings of these subanalyses support the notion that therapeutic approaches should be carefully individualized and tailored for different patients.
Aggressive control of cardiovascular risk factors often associated with ARVD and chronic kidney disease (CKD), such as hypertension, dyslipidemia, metabolic abnormalities, and lifestyle modifications, is part of the current therapeutic arsenal for these patients and will not be discussed in this mini-review. Instead, we will focus on recent strategies aimed to protect the renal parenchyma with translational potential to serve as possible additions to current treatments or as stand-alone therapies.
The degree of functional and structural damage of the renal parenchyma distal to the stenosis may be a critical but often unattended assessment for the selection of therapeutic strategies in ARVD. The kidney is a complex organ in which the vasculature plays multiple roles by providing nutrition of renal tissue, filtration of blood, and removal of systemic waste. Studies from pre-clinical and clinical settings showed that renal microvascular rarefaction, both functional and structural, is a universal pathological feature of CKD that may start early in prominent cardiovascular and renal risk factors like hypertension, diabetes, or obesity and may often predict decline in renal function12–18. Pre-clinical studies also show that ARVD induces a significant and often progressive remodeling and loss of the microvasculature distal to the stenosis, paired with blunted renal mechanisms of microvascular repair and angiogenesis19,20. The progressive microvascular injury is the result of combined insults such as renal ischemia, systemic and renal inflammation, and consequent pro-fibrotic and pro-apoptotic activity that develops in the kidney, often independently of the severity of renal artery stenosis but exacerbated when several cardiovascular risk factors coexist21,22. Moreover, experimental studies showed that PTRAS does not fully recover renal function (despite fully resolving the stenosis) and that microvascular rarefaction and renal fibrosis persist23,24. Furthermore, renal microvascular endothelial cells display poor tolerance to injury paired with limited regenerative capacity, which suggests a distinct environment for early development of renal damage25. All together, these data suggest that the selection of patients suitable for catheter-based interventions should be driven by the anatomic grading of the stenosis and functional assessment of the renal microvasculature since, like the coronary circulation, functional measurements may help to better predict the therapeutic efficacy of PTRAS in ARVD26. In other words, restoration of blood flow into the kidneys may be futile if a severely or irreversibly dysfunctional renal parenchyma is the recipient, and it should not be neglected.
Recent studies support cell-based therapies for the kidney as a feasible option for renoprotection. Intra-renal administration of autologous endothelial or mesenchymal progenitor cells induced significant protection of the renal microvascular architecture (cortex and medulla) that seems to drive functional recovery as well27–30. The effects of cell-based therapies may not be limited to neovascularization or microvascular repair or both. These are pluripotential cells that are mobilized in response to insults in order to build (or restore) pivotal endogenous mechanisms of tissue repair. Indeed, given the diverse cargo of endothelial and mesenchymal cells (for example, extracellular vesicles31–33), it is possible that these cells participate in modulating multiple pathophysiological pathways—such as inflammation, apoptosis, and fibrosis—that may contribute to a steady renal improvement. Thus, this approach represents an excellent opportunity for subsequent exploration of clinical applications in ARVD. Pre-clinical evidence supports their potential, and ongoing clinical trials (for example, ClinicalTrials.gov Identifier: NCT02266394) will determine the safety and efficacy of this strategy for future clinical use.
Targeted strategies for the renal microvasculature are also being tested. Recent experimental studies using intra-stenotic kidney administration of hepatocyte growth factor34 or vascular endothelial growth factor (VEGF)35 in experimental swine ARVD resulted in substantial microvascular repair and neovascularization that correlated with recovery of renal blood flow and filtration function, suggesting that new and repaired microvessels were functional. Furthermore, co-adjuvant VEGF therapy improved the outcomes of PTRAS, emphasizing the notion that the status of the renal parenchyma may play a pivotal role in renal recovery23. Therapeutic angiogenesis for the kidney is a strategy with high potential and is currently under development and refinement. Indeed, ongoing work using drug-delivery technologies to enhance kidney targeting of VEGF in ARVD36–38 and CKD (author’s unpublished work in progress) may boost the translational potential of these strategies.
Another emerging target being considered for renal therapies is the mitochondria. Several forms of acute and chronic renal disease have been associated with changes in mitochondrial homeostasis, formation (biogenesis), morphology, and degradation (mitophagy), and studies show that this plethora of abnormalities may lead to reduced ATP generation, altered calcium signaling, oxidative stress, and cell death39. Although evidence comes mainly from pre-clinical settings (and a few ongoing clinical trials), mitochondrial targeting (via specific inhibition of mitochondrial permeability transition pore opening) shows potential as a feasible strategy to preserve mitochondrial structure and function, restore biogenesis, and ameliorate kidney injury in experimental ARVD (with and without PTRAS40,41), hypertension42, and metabolic syndrome-induced renal damage43. Thus, therapeutic targeting of the mitochondria, being key organelles in cell metabolism and function, could serve as a strategy that induces recovery in the vascular, tubular, and glomerular compartments and has the potential for applications to renal disease of different etiologies and stages.
The development and progression of systemic and renal inflammation are major pathological mechanisms in chronic renal disease of any etiology. Elevation in biomarkers suggestive of inflammation (for example, C-reactive protein, neutrophil gelatinase-associated lipocalin [NGAL], or tumor necrosis factor receptor44), inflammatory cytokines (for example, interleukins and tumor necrosis factor-alpha45), and infiltration of inflammatory cells in the renal parenchyma (for example, neutrophils, lymphocytes, and macrophages46,47) may be observed early, during subclinical stages of CKD, and increase in parallel as CKD advances44. Therefore, biomarkers may be able to evaluate the inflammatory state in kidney disease and help to predict cardiovascular risk. Progressive systemic and renal inflammations are pathological footprints of CKD48 and major determinants for the progression to end-stage renal disease and higher cardiovascular and all-cause mortality49–51. The major etiology of ARVD is atherosclerosis, which is characterized by a chronic low-grade inflammatory state and can instigate inflammatory infiltrates in the kidney49,52. Previous studies aimed to offset inflammation in CKD from diverse etiologies by blocking cytokines that activate, recruit, and are produced by inflammatory cells (for example, macrophages) or by stimulating their depletion50,53. Targeting inflammation has not been tested in ARVD and offers an open field as a novel intervention that warrants further studies.
Chronic inflammation may precede and favor the development of renal fibrosis, a common final pathway of chronic renal diseases irrespective of the etiology. Although fibrosis may be absent, minimal, or at a relatively early stage in ARVD (compared to CKD), the stenotic kidney offers a favorable milieu in which the convergence of insults such as amplified activation of the renin–angiotensin system, oxidative stress, ischemia, inflammation, and microvascular rarefaction may aggravate kidney damage and favor the development of renal fibrosis46,54. Given that renal fibrosis likely is an irreversible stage of loss of functional renal tissue that affects all renal compartments, strategies to prevent or stop its development in ARVD could have a significant impact.
Efforts to develop potential anti-fibrotic strategies for the kidney have been made, with a major focus on anti-transforming growth factor-beta (anti-TGF-β) strategies (for example, inhibition of synthesis, antibodies55,56) but also on other factors such as connective tissue growth factor inhibitors, epidermal growth factor inhibitors, or bone morphogenetic factor agonists57. However, although promising results are observed in pre-clinical studies, conclusive evidence regarding the usefulness of many of the targeted anti-fibrotic strategies is limited, and the understanding of the long-term efficacy and safety of these agents to treat renal fibrosis warrants larger studies. Currently, there are still no clinical therapies in use that specifically target renal cells or kidney fibrosis. Extensive discussion of specific anti-fibrotic strategies and targets is beyond the scope of this brief review, and the reader is encouraged to consult published work57,58.
The kidney is a highly complex organ and therefore therapies are often difficult to fully define. Several tools are available to induce protection of the renal compartments and are part of the standard pharmacotherapy for patients with renal disease. These therapies include inhibitors of the renin–angiotensin system, antioxidants, and lipid-lowering and glucose-control drugs to counteract the deleterious effects on renal function of pathologic conditions that may initiate, accompany, and contribute to the progression of acute and chronic renal disease. Unfortunately, the progressive nature of chronic renal diseases imposes a significant barrier to comprehensive therapies, which should usually be adapted to the patient.
The therapeutic dilemma of ARVD is still a work in progress in which the emphasis placed on resolving renal artery stenosis has overlooked the renal parenchyma, distal to the obstruction (Figure 1). Renoprotective strategies that combine resolution of the stenosis with novel co-adjuvant interventions to preserve, protect, and improve renal outcomes and reduce cardiovascular risk in patients with ARVD should be on the horizon. Efforts should focus on the design and testing of novel comprehensive strategies to protect the renal parenchyma in ARVD, likely in addition to PTRAS. Some novel therapies with translational potential discussed in this brief review are showing promising results in relevant models of ARVD and may change the current paradigm. These are feasible therapeutic alternatives that should be considered and could be tailored to the patient in a timely fashion.
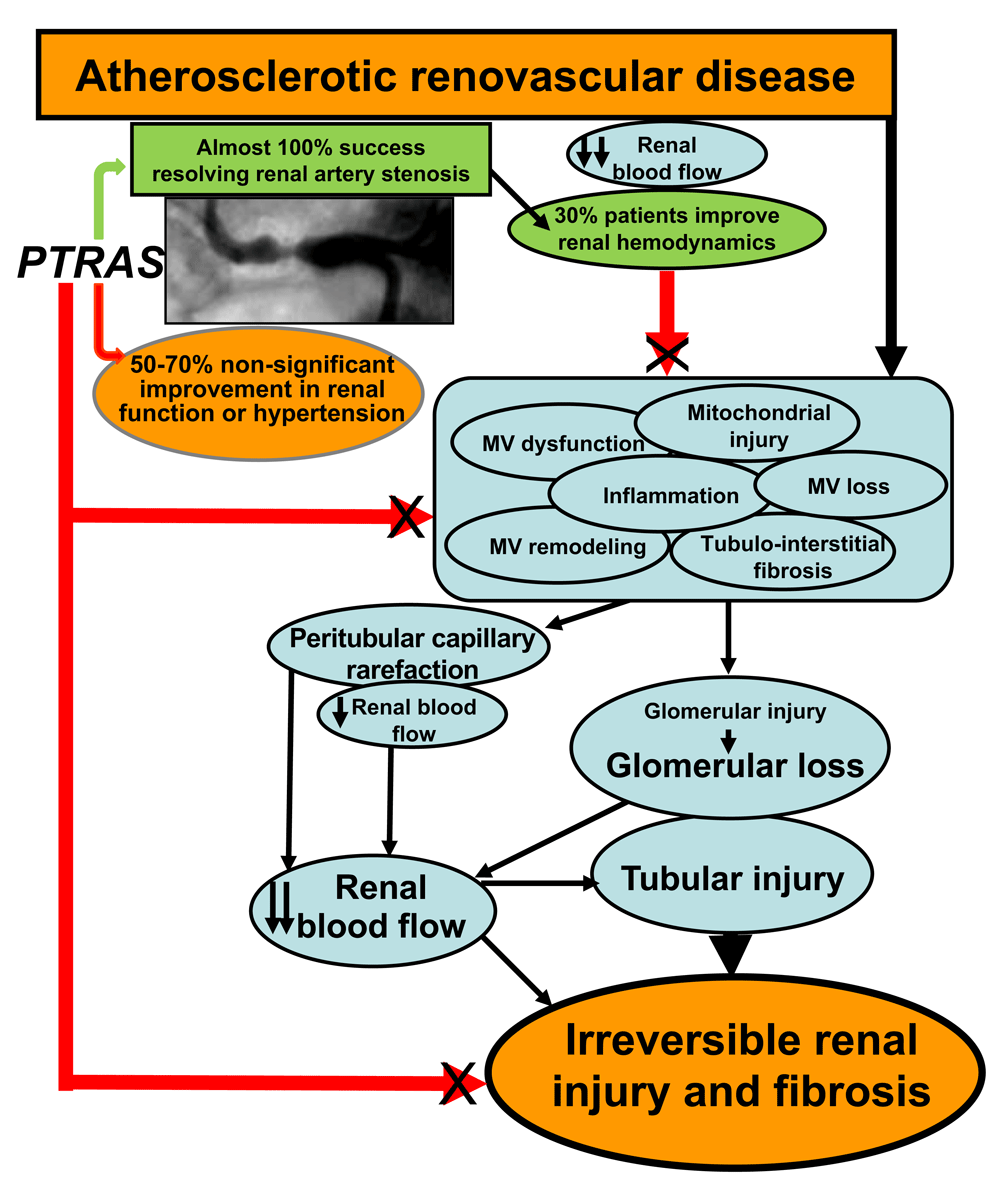
Despite the high success of PTRAS in resolving renal artery stenosis (green arrow and green box), a limited recovery of blood pressure control and renal function is consistently observed (green and orange ovals). This disparity may be due to unresolved progressive pathophysiological mechanisms in the kidney (light blue ovals), distal to the stenosis, that are not addressed by the sole restoration of blood flow by PTRAS (red arrows, black X) and may continue to deteriorate the renal parenchyma. MV, microvascular.
ARVD, atherosclerotic renovascular disease; CKD, chronic kidney disease; PTRAS, percutaneous transluminal renal angioplasty and stenting; RVD, renovascular disease; VEGF, vascular endothelial growth factor
This work was supported by grants HL095638, HL51971, and GM104357 from the National Institutes of Health and grants EIA18490005 and IPA34170267 from the American Heart Association.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 29 Nov 18 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)