Keywords
Cell cell communication, Shh Signalling, retina, development, eye, patterning, regulation
Cell cell communication, Shh Signalling, retina, development, eye, patterning, regulation
The highest functions of the nervous system are based on communication among the huge variety of cells that compose the vertebrate brain. Communication among cells is also fundamental for correct development of the nervous system. Although there are several ways in which neural cells (and cells in general) exchange information, communication mediated by families of signaling molecules such as Wnt, bone morphogenetic protein (BMP), fibroblast growth factor (FGF), and Hedgehog (Hh) is one of the most common. These molecules activate specific signaling pathways that share grossly similar designs, although individual molecular components are specific to each one of the pathways. Ligands are secreted from restricted cellular sources and bind to receptor complexes on the receiving cells. Ligand–receptor binding activates a signaling cascade that ultimately leads to transcriptional regulation of target genes or, less often, to alternative non-transcriptional pathways when more immediate responses are needed. These signaling pathways are used over and over in development to regulate events as diverse as cell specification, proliferation, migration, and differentiation. It follows that their activity needs to be exquisitely controlled, ensuring that information among cells is activated where required and switched off at, or prolonged for, the appropriate time in order to obtain the required context-dependent output. There are different levels of regulation for these signaling molecules. Perhaps the most direct is the existence of classes of secreted proteins that interact with the ligand in the extracellular space, thereby preventing binding to their receptor. This occurs, for example, in the case of BMPs or Wnts, for both of which a large number of secreted antagonists exist1,2. Signaling enhancement also depends on secreted proteins that in some cases promote ligand diffusion as described for Wnt proteins3–6. In contrast, the currently known ligand-binding modulators of the Hh pathway are membrane-bound proteins, prompting the question of whether there is an advantage to such an organization.
Sonic hedgehog (Shh) is the most prominent member of the Hh family in vertebrates and one of the best examples of a classic morphogen7,8, as it induces the acquisition of specific identities in the receiving cells according to the levels and the duration of its signaling9. Shh activates signaling with a mechanism that has been recently defined as “double-negative”10. Indeed, in the absence of the ligand, its 12-pass transmembrane receptor Patched (Ptch) inhibits the seven-pass transmembrane GPCR (G-protein-coupled receptor)-like signal transducer Smoothened (Smo). In the absence of this inhibition, Smo would constitutively maintain the pathway active with the consequent transcription of Shh target genes, mediated by the family of Gli transcription factors. Shh binding to Ptch releases this inhibition and allows the expression of Gli-targeted genes. Gli targets include Ptch itself, thereby establishing a negative feedback loop, important also for limiting ligand dispersion9. Thus, Ptch represses Shh pathway activation by controlling both ligand dispersion and the activity of the signal transducer. In vitro and in silico models have demonstrated that this organization confers robustness to the signaling gradient10 and thus to Shh activity as a morphogen and likely to the additional functions that Shh exerts. So, in principle, there is an advantage to such an organization (see 11 for further discussion). However, activation of Shh signaling is modulated by other surface molecules that either contribute to Shh release from the producing cells, such as Disp (Dispatched)12, or, on the receiving cells, interact with Ptch or Shh or both. The latter include Cdon (cell adhesion molecule-related, downregulated by oncogenes), Boc (Brother of Cdon), Gas1 (growth arrest protein 1)13,14, and Megalin/LRP2 (Megalin/low-density lipoprotein receptor-related protein 2)15. The regulation of the membrane availability of Smo by the tetraspanin Atthog/Mosmo (modulator of Smo) is a recently described additional mechanism of Shh regulation16. Is the presence of these membrane modulators also an advantage?
So far, no studies have formally addressed this question. Nevertheless, in this review, we will use the progressive formation of the vertebrate retina to discuss Shh functions in which some of these regulators have been implicated, pointing to potential advantages and unresolved or controversial issues.
Shh is expressed along the entire axial mesoderm – anterior prechordal plate and posterior notochord – and the ventral midline of the vertebrate neural tube. This distribution prompted the use of the spinal cord as a primary model to understand the mechanism of Shh action17. However, the progressive formation of the vertebrate retina offers an experimental paradigm with which to study how Shh is repurposed to shape multiple developmental aspects of the same structure, from early specification to connectivity.
The eyes are bilateral structures. Their neural component, the retina, originates from a group of cells, known as the retinal field, in the anterior neural plate. As the neural plate folds, cells of the retinal field become displaced laterally, forming two balloon-shaped optic vesicles at the side of the forming neural tube. Shh expression at the prechordal plate is critical for this initial morphogenesis: in the absence of Shh, optic vesicle bilateralism is lost and embryos form, in the most severe case, a single cyclopic eye or, in the milder cases, smaller eyes that are closer together. This phenotype, observed from humans to zebrafish18, is part of a developmental anomaly known as holoprosencephaly (HPE), in which the ventral forebrain is not specified and the dorsal forebrain hemisphere tends to fuse together19,20. In amniotes, there are two concomitant events that contribute to optic vesicle lateralization. The first one is the Shh-dependent specification of the neural plate overlying the prechordal plate into the hypothalamic primordium, which therefore intervenes the two vesicles19. The second is the patterning of the optic vesicles along their proximal–distal axis, which involves the Shh-mediated specification of the proximal/optic stalk domain (reviewed in 17). In teleost fishes, the Shh-mediated posterior-to-anterior migration of medial cells that intercalate into the retinal field is an additional factor21. Genetic inactivation of basic components of the Shh pathway in mouse or zebrafish and mutational screening in patients with HPE confirmed the importance of Shh signaling in ventral central nervous system (CNS) patterning and thus in the proper positioning and growth of the optic vesicles18,22. Similar studies have also shown that Cdon, Boc, Gas1, and LRP2 participate in these developmental events18,23–26.
Cdon and Boc are closely related cell adhesion molecules that can form homophilic and heterophilic complexes and interact with both Shh and Ptch (reviewed in 27). Cdon/Boc interaction with Ptch increases high-affinity ligand binding, indicating their function as Ptch co-receptors and thus as positive signaling regulators14,23,28–30. The two genes are expressed with largely overlapping patterns that include the entire dorsal neural tube and the developing eye and ear and the olfactory system31,32. This distribution often coincides with that of Gas133, encoding a GPI (glycosylphosphatidylinositol)-linked protein that also interacts with Shh and Ptch34,35 (Figure 1A). Mouse embryos lacking Cdon, Boc, and Gas1 show a phenotype that mimics Shh loss of function19, which leads to the absence of the entire ventral neural tube resulting in severe HPE and early embryonic lethality (Figure 1C). This indicates that the three co-receptors play positive and overlapping roles in regulating Shh pathway activation13,14. Furthermore, Shh signaling represses Cdon, Boc, and Gas1 expression30,36. This suggests that these co-receptors may serve as buffers to prevent possible defects due to abnormally low Shh signaling because, if Shh activity decreases, their upregulation could boost signaling again. However, genetic inactivation of the individual co-receptor genes reveals non-equivalent roles. Gas1 null mouse embryos present ventral neural tube defects, mild HPE, and mis-specification of the ventral retinal pigmented epithelium into a neural retina-like tissue33,36. Cdon null embryos display a similar mild HPE (Figure 1B) with small eyes and coloboma (opened optic fissure)23,37. Boc null mice instead have none of these defects but, when crossed with either Cdon or Gas1 mutants, enhance their respective HPE phenotype24,25. Whether Boc also modifies their respective specific eye phenotype remains to be studied. Somewhat in line with these differences, systematic genomic sequencing analysis of patients with HPE has identified causative mutations in the CDON gene23,38 but only sequence variations suggestive of a modifier role for BOC and perhaps for GAS138. Given that these co-receptors have all been shown to foster Shh signaling, it is not obvious why their loss of function causes these phenotypic differences, especially in the case of the closely related Cdon and Boc. One possibility is that Shh signaling exerts a differential negative regulation on their expression. Alternatively (or additionally), Cdon and Boc may employ distinct mechanisms to enhance Shh signaling, as recently suggested29. For example, the ectodomain of Boc, but not that of Cdon, can be proteolyzed29. If this proteolysis occurs in vivo, which is still a matter of speculation, Boc ectodomain could enhance Shh diffusion and at the same time terminate high-affinity binding of Shh to Ptch. The two effects may compensate one another, explaining the lack of HPE phenotype in Boc mutants. Variations in Cdon, Boc, and Gas1 distribution may also underlie the observed differences in the mutants’ phenotype. This differential expression may also offer an alternative explanation for how Cdon and Boc influence Shh signaling. Indeed, whereas the ventral neural tube and optic vesicle expression of Gas133 makes it easy to understand its Ptch co-receptor function, the predominant dorsal expression of Cdon and Boc31,32 makes the same function less immediately understood. Boc and Cdon could be transiently expressed in the ventral neural tube right when needed for early patterning, as reported for the zebrafish Boc orthologue39. However, the expression of Cdon, but not Boc, in the axial midline of both mouse and zebrafish30,40 suggests that Cdon could have the additional role of favoring Shh release from the producing cells. The Drosophila homologue of Cdon, interference Hh (ihog), has been reported to have such an activity41, although motif differences between Shh and its Drosophila homologue Hh call for caution in applying directly to vertebrates what has been learned in the fly42. Nevertheless, the HPE phenotype of Cdon null embryos could easily be explained by an attenuated Shh release from the midline, a function in which Boc may not be implicated.
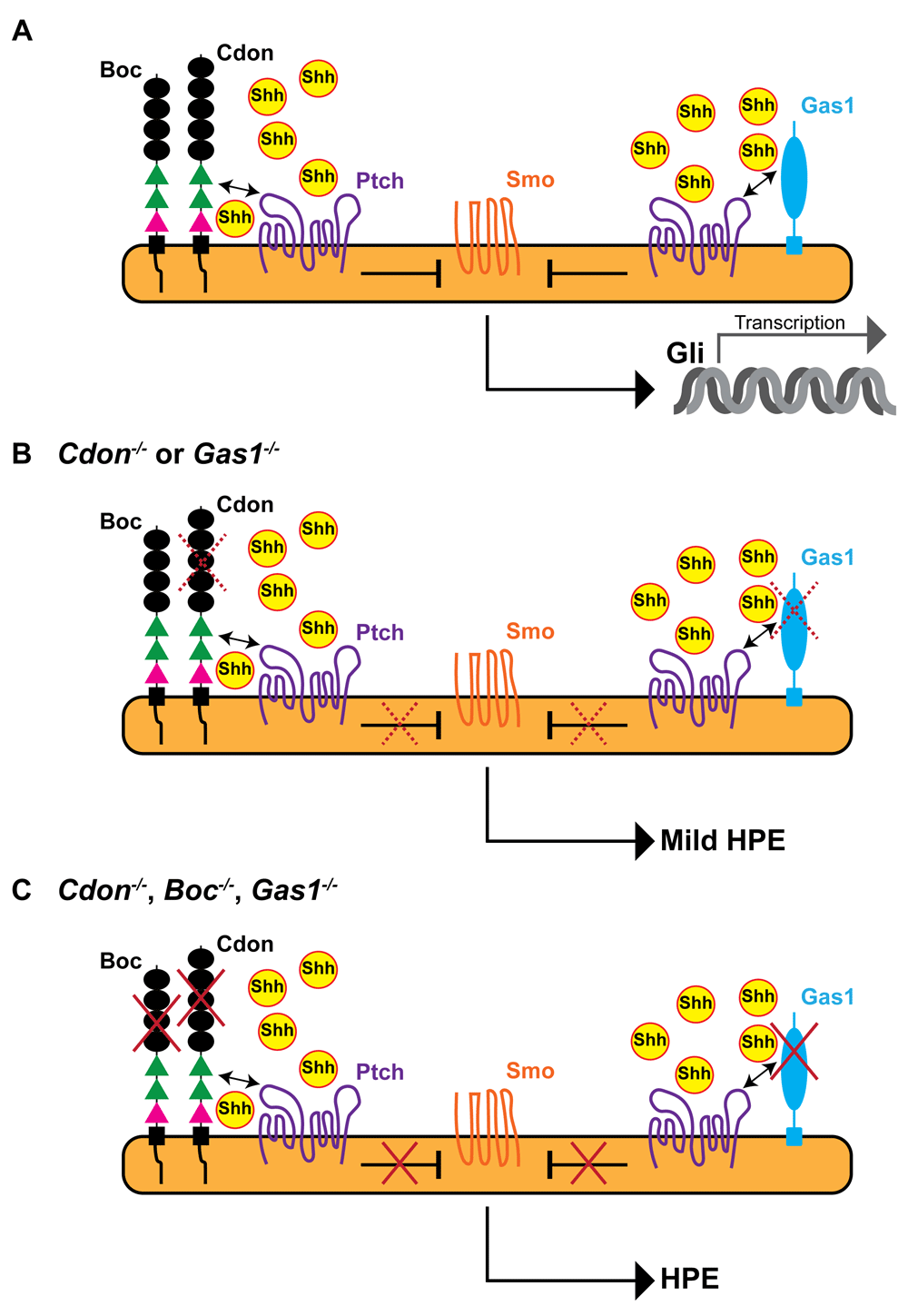
The diagrams represent the interaction of Cdon, Boc, and Gas1 with Ptch and Shh during Shh-mediated patterning of the ventral neural tube in wild-type embryos (A) or in embryos with genetic inactivation of either Cdon or Gas1 function (B) or lacking Cdon, Boc, and Gas1 (C). The three co-receptors interact with Ptch and the complex binds Shh with high affinity. In the presence of Shh, Smo is de-repressed (red crosses) and activates a signal transduction cascade that culminates with Gli-mediated transcription of Shh target genes. The Cdon/Ptch and Boc/Ptch interactions are mediated by the FnIIIa and FnIIIb domains (green) of Cdon and Boc, respectively. Binding of Shh to Cdon or Boc is mediated by the FnIIIc domain (pink). (B) In the absence of either Cdon or Gas1, Shh is less activated (dotted red crosses), resulting in mild craniofacial defects. (C) Loss of all three co-receptors prevents pathway activation, resulting in severe HPE, a phenotype that mimics Shh loss of function. Boc, Brother of Cdon; Cdon, cell adhesion molecule-related, downregulated by oncogenes; Gas1, growth arrest protein 1; HPE, holoprosencephaly; Ptch, patched; Shh, sonic hedgehog; Smo, smoothened.
At the moment, this is only a hypothesis but it may be worth testing. It is equally unexplored whether LRP2 can functionally interact with Boc, Cdon, or Gas1 or with their possible different heterodimeric or trimeric complexes. LRP2 facilitates Shh/Ptch binding and promotes the internalization of the complex, which is required to relieve Smo inhibition. Thus, in the absence of LRP2, Shh signaling is impaired, leading to embryos with an HPE phenotype43. It remains an open question whether LRP2 promotes Cdon, Boc, and Gas1 internalization when bound to Ptch or instead competes with them for Ptch and Shh binding.
The work we have discussed so far, independently of the still-puzzling aspects, supports a positive role of Cdon, Boc, Gas1, and LRP2 in Shh signaling and thus in the specification of the ventral CNS and eye separation. However, Cdon, Boc, and LRP2 have been shown to act as negative regulators of Shh signaling as retinal development progresses, although each one of them does so in different contexts (Figure 2).
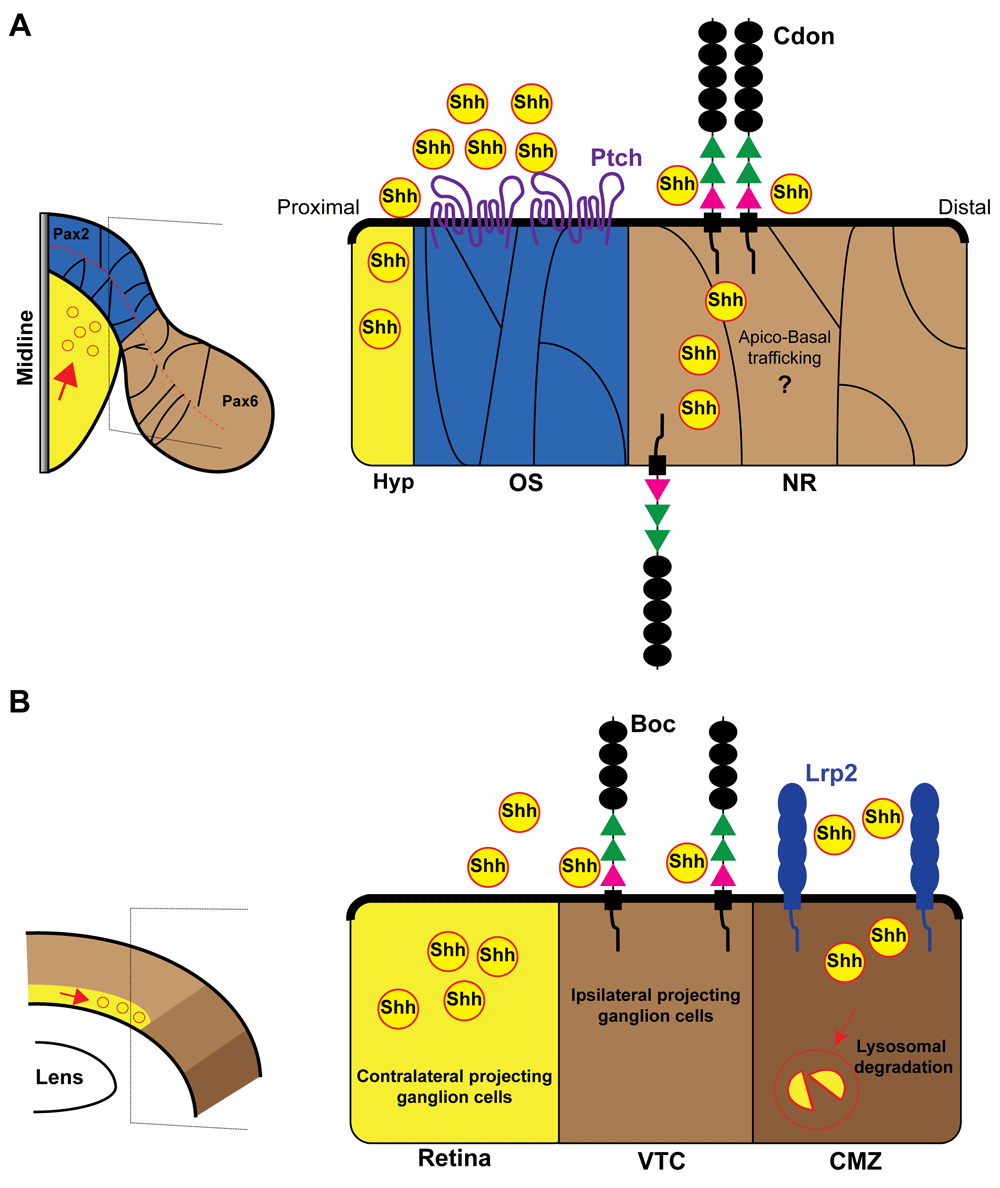
(A) Schematic dorsal view of the optic vesicle (left) and enlarged view of the optic stalk/neural retina border (right). The expression domains of Pax2 (blue) and Pax6 (brown) are indicated in the scheme. At the border of these two domains, Cdon binds Shh, serving as a decoy receptor to protect the neural retina from midline-derived Shh activity. Note that the Ptch receptor localizes only in the Pax6-positive neural retina domain. (B) Schematic frontal view of mature retina (left) and enlarged view of the retinal periphery (right). Contralateral RGCs produce and secrete Shh. Ipsilateral projecting RGCs express the co-receptor Boc that prevents Shh diffusion and thus signal activation. Low Shh signal allows for the specification of ipsilateral program specification in RGCs of the VTC. Lrp2/Megalin instead limits Shh proliferative activity by endocytic clearance of Shh at the CMZ. Boc, Brother of Cdon; Cdon, cell adhesion molecule-related, downregulated by oncogenes; CMZ, ciliary marginal zone; Hyp, hypothalamus; Lrp2, low-density lipoprotein receptor-related protein 2; NR, neural retina; OS, optic stalk; Pax2, paired box protein Pax-2; Pax6, paired box protein Pax-6; Ptch, patched; RGC, retinal ganglion cell; Shh, sonic hedgehog; VTC, ventrotemporal crescent.
As mentioned before, the formation of two bilateral optic vesicles implies the compartmentalization of its neuroepithelium in different domains along the different axes. One of the first subdivisions occurs along the proximo–distal axis of the vesicle and originates the prospective optic stalk proximally and the prospective retina distally (Figure 2A). The establishment of the optic stalk and retinal domains is defined by the specific and respective expression of two paired- and homeobox-containing transcription factors: paired box protein Pax-2 (Pax2) and Pax6 (reviewed in 44). The two factors cross-repress each other and thus define a sharp border between the two territories45 (Figure 2A). Shh signaling promotes Pax2 expression, thereby imposing optic stalk identity. When Shh is reduced or absent, the optic stalk domain is smaller or absent and the two retinal domains tend to fuse together. Shh overexpression has the opposite effect with an excess of Pax2-positive optic stalk that overtakes the retinal domain by repressing Pax617,44. This means that the right amount of Shh signaling is critical to form a precise boundary between the optic stalk and the retina. Recent studies have shown that, at least in zebrafish and chick embryos, Cdon participates in the establishment of this boundary40. In both species, ptch is expressed in the pax2-positive optic stalk, whereas cdon, but not boc, is strongly expressed in the presumptive neural retina overlapping with pax6 distribution (Figure 2A)40. The complementarity between ptch and cdon expression advocates against a synergistic role. Indeed, morpholino-mediated knockdown of cdon allows for the expansion of the optic stalk, decreases eye size, and prevents optic fissure closure, indicating that Cdon counteracts Shh effect40. This phenotype depends on the ability of Cdon to bind Shh but not Ptch. Furthermore, it is a direct consequence of Cdon activity in the retina because targeted cdon overexpression in the zebrafish retina is sufficient to rescue the phenotype of cdon knockdown and spatiotemporal restricted interference with Cdon retinal expression in chick embryos mimics the zebrafish phenotype40. The precise mechanism by which this happens is still unrefined; however, when mis-expressed close to the optic recess midline (a Shh source), Cdon binds Shh with great efficacy and serves as a sink to limit ligand availability to the nearby cells40. This indicates that Cdon acts as a decoy receptor to protect the neural retina from Hh activity (Figure 2A).
A similar function has been postulated for Boc during mouse retinogenesis46. Retinal ganglion cells (RGCs) are the first neurons to be born in the retina of all vertebrates. Newly generated RGCs express Shh, and this expression promotes the propagation of RGC specification and differentiation, the proliferation of retinal precursors, and their differentiation toward other neuronal cell types (reviewed in 47,48). In the mouse, a small proportion of RGCs located in the ventrotemporal crescent of the retina do not express Shh49 (Figure 2B). These neurons are special because, in contrast to all the Shh-positive RGCs, they project to the ipsilateral side of the brain, enabling the semi-binocular vision typical of rodents. These ipsilateral RGCs express Boc49,50. In these neurons, Boc is necessary to keep Shh signaling low, thereby enabling the expression of the transcription factor Zic246, a determinant of the ipsilateral program51. Thus, in Boc null mice, part of ipsilateral RGCs are mis-specified, acquiring a contralateral projecting phenotype with a consequent alteration of the retinal projections46. In an additional and not necessarily contrasting view, Boc, present on the membrane of ipsilateral RGC growth cones, mediates guidance information provided by Shh at the optic chiasm midline, forcing the axons to enter the ipsilateral optic tract50. Notably, Shh, transported along the axons of the contralaterally projecting RGCs49,52, seems to be released at high concentrations and with a still-unknown mechanism (see 53 for discussion), right at the chiasm providing Boc-mediated repulsive information to ipsilateral axons52. Thus, in this case, Boc would act as a positive mediator of Shh. Whether the same molecule can have a double function in the same cell remains to be established, but, in a speculative view, Boc interactions at the perikaryon could be different from those existing at the growth cone.
Independently of this still-unanswered question, both Cdon and Boc can function as negative regulators of Shh activity, limiting ligand dispersion, a function that has been observed in Drosophila wing disc and ovary development41,54,55. Incidentally, both Cdon and Boc, when ectopically expressed close to a Shh source, localize predominantly at the basal side of the neuroepithelial cells, where they accumulate most of the bound Shh protein40. A recent study revisiting the function of Hhip (Hh-interacting protein)—initially defined as a membrane-bound negative regulator of HH signaling56—showed that Hhip is secreted and localizes to the neuroepithelial basal membrane57. The basal localization of both Cdon and Hhip is interesting because it may serve to clear the ligand from the apical surface of the neuroepithelial cells, where the primary cilium localizes. This organelle is fundamental for Shh signal transduction, as it hosts the main components of the transduction machinery of this pathway58.
A negative regulation of Shh signaling, based on a different mechanism of ligand clearance, has also been proposed for LRP2. Though initially expressed in the whole optic cup, LRP2 expression becomes restricted to the peripheral margin as retina differentiation proceeds. This region, called the ciliary marginal zone (CMZ), is a source of progenitor cells in fish and amphibians59 and likely also in the mammalian embryonic retina60,61. The CMZ is normally devoid of Shh activity. Deficiency of Lrp2 in mice or zebrafish causes enlarged and exophthalmic eyes62–65, a pathological condition known as buphthalmos and observed in patients carrying LRP2 mutations66. Searching for an explanation for this phenotype, Christ et al.65 found elevated transcript levels for GLI family zinc finger 1 (Gli1) and Ptch1 genes, two Shh targets, suggesting that LRP2 protects the CMZ from the influence of RGC-derived Shh. In the absence of LRP2, Shh induces CMZ progenitor hyperproliferation, expanding the overall eye size. Mechanistically, LRP2 mediates lysosomal clearance of Shh alone, thereby maintaining the CMZ quiescent65 (Figure 2B).
Although their local function has not been explored in detail, Cdon, Boc, and Gas1 are also strongly expressed in the CMZ36,40,49, making this structure an attractive model to study possible interaction among all these Shh-binding proteins. In a speculative view, they could all concur to make the CMZ a Shh-free zone given that Gas1 has also been initially proposed to work as a Shh sink34.
In conclusion, going back to the original discussion point, “economy” could be the main advantage of controlling potent signaling molecules with membrane-bound proteins. Cdon, Boc, and Lrp2—in the specific case of Shh—seem to act as both positive and negative regulators of the signaling pathway depending on their additional interaction with other membrane-bound proteins (Ptch). In a speculative view, an economical way of changing the role for these regulators from a positive to a negative one might be their shuttling from the apical to the basal membrane. The destiny of Shh bound to Boc or Cdon when these proteins act as negative regulators is a matter of speculation. However, in the same economical view, unwanted Shh could be recycled back to the tissue where it is needed, such as from the neural retina to the optic stalk. The observed redundancy of regulatory molecules may not fall into the view of economy, although redundancy might be the best way of ensuring the needed levels of Shh during development and homeostasis.
Bmp, bone morphogenetic protein; Boc, Brother of Cdon; Cdon, cell adhesion molecule-related, downregulated by oncogenes; CMZ, ciliary marginal zone; CNS, central nervous system; Gas1, growth arrest protein 1; Hh, hedgehog; Hhip, hedgehog-interacting protein; HPE, holoprosencephaly; LRP2, low-density lipoprotein receptor-related protein 2; Pax2, paired box protein Pax-2; Pax6, paired box protein Pax-6; Ptch, patched; RGC, retinal ganglion cell; Shh, sonic hedgehog; Smo, smoothened
Work in our lab is currently supported by the following grants: MINECO BFU2016-75412-R with FEDER funds, BFU2016-81887-REDT, and PCIN-2015-176-C02-01/ERA-Net NeuronII; Fundación Ramón Areces-2016 and CIBERER, ISCIII (to PB). VG holds H2020-MSCA-IF-2016 grant 740916. We acknowledge an Institutional CBMSO Grant from the Fundación Ramón Areces.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 14 Dec 18 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)