Keywords
androgen deprivation therapy, metastasis, PSA kinetics, prostate cancer, survival, systematic review, meta analysis
androgen deprivation therapy, metastasis, PSA kinetics, prostate cancer, survival, systematic review, meta analysis
Prostate cancer (PCa) is the second most common cancer in men, and the fourth most common cancer worldwide. More than one million men worldwide were diagnosed with PCa in 20121. The incidence of local-regional PCa has increased since the introduction of prostate specific antigen (PSA). This circumstance reduces the incidence of metastatic PCa2. PCa patient treated at early stages have a good prognosis with 5-year overall survival (OS) reaching 99%. In contrast, metastatic PCa patients generally experience a poor outcome. Several published studies showed a wide difference of survival, with median OS from two to five years3–5. Androgen deprivation therapy (ADT) becomes the standard treatment of patients with advanced PCa6,7, and with the first use reported by Huggins and Hodges in 19418.
In clinical practice, PSA is the most common diagnostic procedure to evaluate the disease and to predict the survival. PSA kinetics such as nadir PSA level, time to reach nadir (TTN), or specific PSA value after initiation of ADT might became a predictor of survival in several retrospective and clinical trial studies5,9–11. Some limitations were shown in the previous report of investigation for PSA kinetic to survival. They included patients with heterogeneous backgrounds (such as metastatic disease prior to surgical or radiation therapy), and the sample size was small. Therefore, we performed a systematic review and meta-analysis to evaluate the pooled effect of PSA kinetics of patient treated with hormonal therapy related to survival from several published studies.
The systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines12. All studies in English were included. Retrospective cohorts, prospective cohort, randomized clinical trial (RCT), were eligible for inclusion for this review. The inclusion criteria were that (i) the participant of the study had metastatic PCa; (ii) patients were treated with ADT either using orchiectomy or luteinizing hormone-releasing hormone (LHRH) agonist with or without anti-androgen (AA); (iii) the studies outcome were either progresion free survival (PFS), overall survival (OS) or cancer specific survival (CSS); (iv) the studies had to analyze PSA kinetics (intial PSA prior to initiation of ADT, PSA nadir, and time to reach nadir (TTN) PSA). Studies analyzed in meta-analysis had to use adjusted analysis of prognostic factors, such as multivariate Cox regression, to overcome the confounding factors. Studies that analyzed patient with castration resistant PCa (CRPC) and non-metastatic disease were excluded.
Electronic searched were performed in three databases: MEDLINE, Science Direct, and Cochrane Library from 1950 to 2016. This literature searching was conducted in March 2017. Gray literature and conference abstract, especially from urology oncology conference, were also searched. References list from included article were reviewed. We used the following search strategy: (prostate cancer OR adenocarcinoma prostate), (survival OR prognosis OR prognostic), (metastasis OR metastases OR metastatic), (PSA OR “Prostate Specific Antigen” OR nadir OR “initial PSA” OR kinetic). Two researchers (A.A and A.R.A.H) were indecently assessing the title and abstract of the paper. They agreed the studies included in the meta-analysis. Disagreement between the two review authors on the selection of studies was resolved by discussion with third authors (C.A.M) as a senior investigator. We used EndNote X6 for screening of duplicated studies.
A data extraction table was created to extract data from each article. The data of study design, patient's characteristics, method of ADT, duration of follow up, outcomes of survival, and significant prognostic factors of PSA kinetics were collected from all included studies. For the observational studies, the quality of study was assessed using Newcastle-Ottawa Scale (NOS). There were three major components of this scale namely the selection of the group of the study, comparability, and assessment of the outcome. The quality of study assessed with number of stars based on NOS. A maximum 7 stars could be scored; 6 or 7 stars considered as high quality study, 4 – 5 stars corresponded with intermediate quality, and 0 – 3 stars showed low quality13.
Meta-analysis was applied on studies with prognostic factor with similar outcome definition. I2 test was conducted in order to evaluate the heterogeneity, whilst for >30% a random effects model was applied, or otherwise, fixed effects model was done. Confounding in the individual studies was estimated using Hazard Ratio (HR) adjusted estimation, thus generic inverse variance method was used. We only combined data to estimate pooled effect of categorical parameters due to feasibility of statistical analysis. Studies that evaluated parameters but could not synthesize to meta-analysis were describe quantitatively. Meta-analysis was performed using Review Manager 5.2 from Cochrane Collaboration. A p-value < 0.05 was considered statistically significant.
We found 873 citations throughout database searching. No additional records identified through searching from reference list of included studies. Seventeen studies were found to be consistent to the inclusion criteria of the study, but seven studies could not be evaluated the in meta-analysis (Figure 1). Miyamoto et al.14 did not published the hazard ratio, and put the cumulative survival rate as the outcome. Six other studies used numerical parameters of PSA kinetics that cannot combine in the forest plot9,15–19. All of those studies were considered in qualitative synthesis. The characteristic of study is present in Table 1. Based on NOS, the quality of study included was good (Table 2).
| Study | Total patient | Androgen Deprivation Therapy | Follow - up time | Survival outcome | Significance Prognostic Factor |
|---|---|---|---|---|---|
| Bello 201725 | M1 = 79 | • Orchiectomy • LHRH agonist | NM | Median OS was 40.3 months | • NSAID use • PSA nadir |
| Choueiri 20095 | M1 = 179 | • LHRH agonist with or without AA • Orchiectomy | Median follow up 48 months | Median OS was 84 months | • GS • TTN • PSA Nadir |
| Glass 20039 | M1 = 1,076 | Bilateral orchiectomy with or without AA (flutamide) | NM | Median OS was 32 months | • Initial PSA • Presence appendicular bone disease • GS • Presence of bone pain • PS |
| Hong 201210 | M1 = 131 | CAB using LHRH agonist plus AA | Median follow up 30 months | Median CSS • PSA nadir < 2 ng/ml was 91.7 months • PSA nadir ≥ 2 ng/ml 49.8 | • PSA nadir • TTN |
| Hussain 200611 | M1 = 1,345 | CAB using LHRH agonist (gosereline) plus AA (bicalutamide) | Median follow up was 38.0 months | Median OS of PSA after 7 months ADT • ≤ 0.2 ng/ml was 75 months • 0.2 < PSA ≤ 0.2 ng/ml was 44 months • PSA > 4.0 ng/ml was 13 months | • PSA after 7 months of ADT • ECOG • Presence of bone pain • GS |
| Kadono Y, 20154 | M1a = 224, M1b = 4386, M1c =278 | • LHRH agonist with or without AA • Orchiectomy | Mean follow up 3.3 years | The 5-year OS was • 57.5% in M1a • 54.0% in M1b • 40.0% in M1c | • GS • PSA • Age |
| Kim KH, 201518 | M1 = 398 | • CAB (LHRH agonist plus AA) | Median follow up 44 months | Median CSS was 65 months | • GS • PSA nadir • TTN • PSA Half Life • N1 |
| Kimura, 201424 | M1 = 3006 | • Type of ADT was not clear | Median followed up in young, middle and elderly group was 25.5, 35.3 and 38.5 months | The 5-years OS • Young age was 26.6% • Middle age was 59.7% • Elderly age was 55.3% | • GS • Concomitant bone and visceral metastasis • Age • Clinical T |
| Koo, 201417 | M1b = 248 | • Type of ADT was not clear | Median follow up 39.3 months | Median CSS in PSA nadir • < 0.2 ng/ml was 70 months • ≥ 0.2 ng/ml was 50 months | • PSA nadir • ALP • ECOG |
| Kwak 200223 | M1 = 145 | • LHRH agonist with or without AA • Orchiectomy | Median follow up 39 months | Median survival of patients with Nadir PSA (months) • < 0.2, = 53 • 0.2 to 1.0 = 42 • 1.1 to 10 = 24 • >10.1 = 15 | • Nadir PSA |
| Miller 199225 | M1 = 48 patients | • Orchiectomy • LHRH agonist • Diethylatibestrol | Median follow up 42 months | Median PFS 19 months | • Nadir PSA |
| Miyamoto 14 | M1 – 94 | • LHRH agonist with AA | Median follow up 38.8 months | 5-yr OS rate 62.5% | • PSA • Gleason Grade |
| Nayyar 2010 16 | M1 = 412 | • Surgical castration • Medical castration • Antiandrogen | Median follow up 55 months | Median OS 5.7 years | • GS • PSA doubling time |
| Park, 200915 | M1 = 131 | • LHRH agonist with or without AA • Orchiectomy | Median follow up was 53.0 months | Median CSS • Short PSA doubling time was 35 months • Long PSA doubling time 95 months | • High Nadir PSA • Short PSA half time • Short PSA doubling time after nadir |
| Sasaki 2011 22 | M1 = 412 | • Bilateral orchiectomy • LHRH agonist | NM | Median OS 5.7 years | • PSA half time • PSA doubling time • GS |
| Teoh 2017 21 | M1b = 419 | • LHRH agonist • Bilateral orchiectomy | Median follow up was 48 months | Median OS was 28 months | • PSA nadir ≥ 2 ng/ml • TTN < 9.09 months |
| Tomiokoa 201420 | M1 = 236 | • LHRH agonist • surgical castration • AA monotherapy • CAB | Median follow up 47 months | The 5-years OS was 63% | • Nadir PSA ≥ 0.2 ng/ml • TTN < 6 month |
ADT = androgen deprivation therapy; LHRH = luteinizing hormone releasing hormone; AA = anti androgen; CAB = combined anti androgen; PSA = prostate specific antigen; OS = overall survival; GS = Gleason score; TTN = time to nadir; NM = not mentioned; NSAID = non-steroidal anti inflammatory drug; ECOG PS = Eastern Cooperative Oncology Group Performance Status; PS = Performance Status; ALP = alkaline phosphatase; N1= regional nodal metastasis
| Study | Selection (Max ****) | Comparability (Max **) | Outcome (Max ***) | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of exposed cohort | Ascertainment of exposure | No outcome of interest at start | Comparability of cohorts on the basis of design of analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Bello 201725 | * | * | * | * | ** | * | Not clear | Not clear | 7 |
| Choueiri 20095 | * | * | * | * | * | * | * | * | 9 |
| Glass 20039 | * | * | * | * | ** | * | Not clear | Not clear | 7 |
| Hong 201210 | * | * | * | * | ** | * | * | * | 9 |
| Hussain 200611 | * | * | * | * | ** | * | * | * | 9 |
| Kadono 20154 | * | * | * | * | ** | * | * | * | 9 |
| Kim 201518 | * | * | * | * | ** | * | * | * | 9 |
| Kimura 201424 | * | * | * | * | * | * | * | * | 8 |
| Koo 201417 | * | * | * | * | ** | * | * | * | 9 |
| Kwak 2002 | * | * | * | * | ** | * | * | * | 9 |
| Miller 199219 | * | * | * | * | * | * | * | * | 8 |
| Miyamoto 201214 | * | * | * | * | ** | * | * | * | 9 |
| Nayyar 2010 | * | * | * | * | ** | * | * | * | 9 |
| Park 200915 | * | * | * | * | ** | * | * | * | 9 |
| Sasaki 201122 | * | * | * | * | ** | * | - | * | 7 |
| Teoh 201721 | * | * | * | * | ** | * | * | * | 9 |
| Tomioka 201420 | * | * | * | * | ** | * | * | * | 9 |
Initial PSA. Initial PSA before ADT treatment was evaluated in twelve studies4,9–11,16,17,19–25. However, we only put four studies in PFS outcome and four studies in CSS/OS outcome because the studies analyzed initial PSA as a categorical parameter. No significant association between initial PSA and PFS was found, and the studies were homogenous (I2=0%). In addition, there was no association between initial PSA and CSS/OS (Figure 2). In qualitative analysis, four studies analyzed the association between initial PSA and PFS. All of the studies did not find significant results for PSA and PFS16,17,19,25. The result was the same when we analyzed the studies for OS/CSS outcome9,11,16.
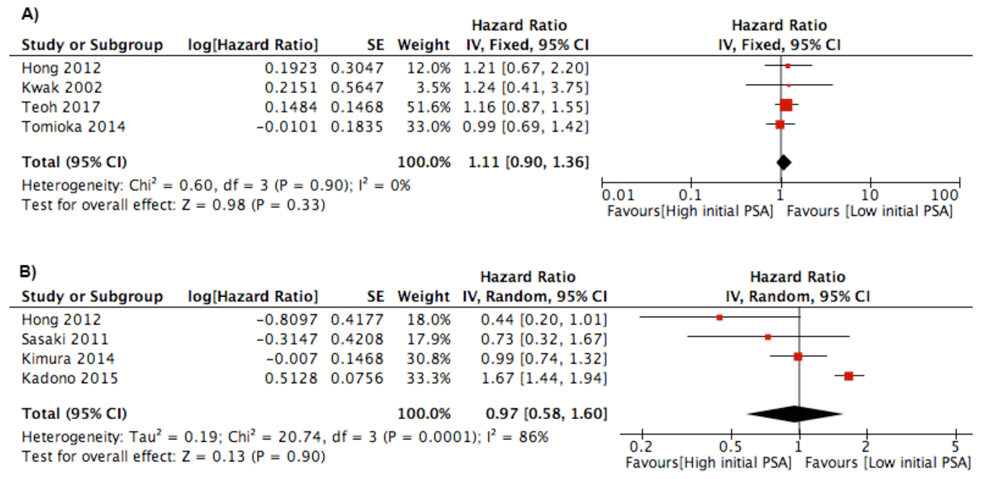
Forest plot of association between initial PSA and: A) Progression Free Survival Outcome; B) Cancer Specific Survival/Overall Survival.
PSA Nadir. Six studies analyzed the effect of PSA nadir to influence survival using 0.2 ng/ml as a cut-off point5,10,11,20,22,23. Four studies analyzed the PSA nadir as a continuous variable15,17–19. Teoh et al. used cut-off point 2 ng/ml as a PSA nadir that influence the survival21. Bello et al. analysed nadir using 4 ng/ml as a threshold25. Meta-analysis of the studies found an association of reduced PFS of patient with high PSA nadir (HR 2.22; 95% CI 1.82 to 2.70). The studies appear homogenous in the forest plot. In addition, high PSA nadir had a negative impact on the OS/CSS outcome with HR 3.31 (95% CI 2.01–5.43) (Figure 3). In the studies using continuous measurement of PSA nadir, three studies found significant association of nadir PSA and survival15,18,19. However, studies by Koo et al. found no significant result17. Miyamoto et al. found the PSA nadir after first line hormonal therapy influenced survival14.
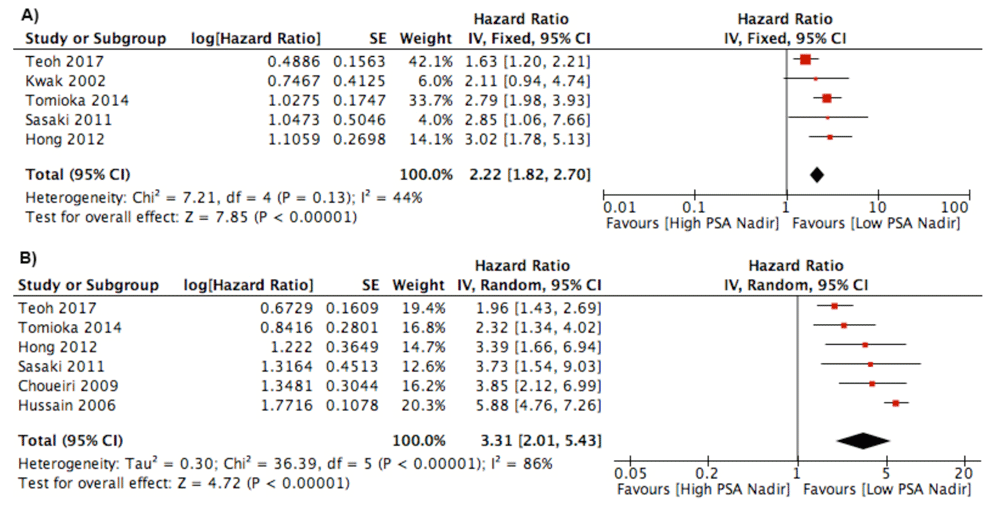
Forest plot of association between PSA nadir and: A) Progression Free Survival Outcome; B) Cancer Specific Survival/Overall Survival.
Time to Nadir (TTN). A total of seven studies analyzed the relationship between TTN and survival5,10,16–18,20,21. Of the seven studies, two studies used 8 months5,10, one study used 9 months26, and one study used 12 months20 as a cut-off. Three studies analyzed TTN as a continuous variable16–18. Meta-analysis was performed with showing a shorter TTN correlated with poor survival for both PFS (HR 2.41; 95% CI 1.19 – 4.86) or CSS/OS (HR 1.80; 95%CI 1.42 – 2.30) (Figure 4). Studies using continuous variable of TTN showed a significant negative effect from shorter TTN on survival.
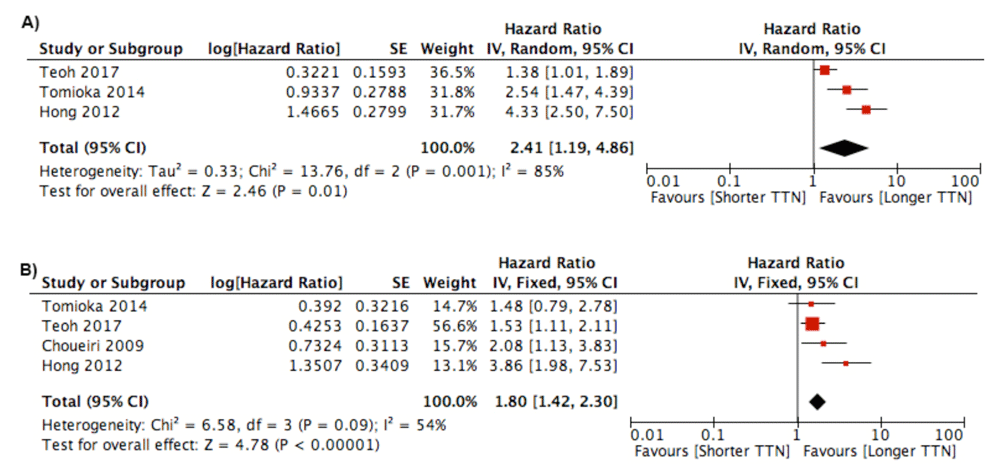
Forest plot of association between TTN and: A) Progression Free Survival Outcome; B) Cancer Specific Survival/Overall Survival.
Nowadays, clinicians have use PSA not only for screening for PCa, but also for follow up of patients after the treatment. The PSA indicates PCa condition following radical treatment in localized disease, and hormonal treatment in metastatic condition. PSA has a prognostic value, and now has been widen to several parameters such as PSA nadir, TTN, PSA doubling time, and PSA response after the treatment. There is controversy among previous study about the utilization of the PSA kinetic after hormonal treatment for predicting the progression to CRPC and survival.
The meta-analysis performed in this study did not find an association between survival and high initial PSA. Significant heterogeneity was observed due to scattered cut off points of high initial PSA amongst the studies included. Several studies found significant association of initial PSA and survival in univariate analysis, but lost significant after multivariate analysis. This condition showed us the aggressiveness of the cancer has not reflected by PSA alone, and other measures such as Gleason score, PSA nadir, and PSA decline may need to be considered10,15. This finding was different in localized diseases. High initial PSA reflects disease burden and was found to be correlated with the pathological stage, Gleason score, and the risk of metastasis. The National Comprehensive Cancer Network (NCCN) guideline stratified the risk of localized disease based on PSA and that influences the treatment choice27.
The significant findings of this study showed that lower PSA nadir was associated with good prognosis after ADT treatment. However, due to the variety of PSA nadir threshold, we could not conclude the best optimal threshold of PSA nadir. Most of the papers in this meta-analysis were using below 0.2 ng/ml PSA nadir. Morote et al. analyzed 185 patients with metastatic prostate cancer and they found nadir PSA above 0.2 ng/ml was associated with 20 times likelihood progression to CRPC28. Moreover, Stewart et al. analyzed patient who received ADT due to biochemical recurrence after radical prostatectomy or radiation therapy, and they suggested PSA nadir above 0.2 ng/ml was associated with significant progression and mortality29. Keizman et al. used a different cut off for PSA nadir. They were using below 0.1 ng/ml because they found 4 times increased likelihood of biochemical or clinical progression in patients treated with intermittent ADT due to relapse after radical treatment30.
Our findings found an association between longer time to get nadir PSA and survival. Longer time to nadir was associated with good prognosis. A study by Chung et al. found longer time to achieve nadir was a good prognosis for postoperative or post-radiation failure patients receiving ADT31. Possible mechanisms of longer time to nadir associated with a good prognosis was associated with differentiation of PCa cells. Rapid reduction of hormone sensitive cancer cells may induce an environment for the development of hormone resistant PCa cells. In addition, PCa cells that have potential to differentiate into castration resistant cell show a rapid reduction of PSA due to ablation of the androgen receptor. Thus, rapid reduction of PSA is associated to development of CRPC and has a poor prognosis32. This phenomenon is opposite to organ confined PCa receiving radical prostatectomy. In this setting, rapid decline of PSA result is associated with a better prognosis33.
This study has some methodological limitations. We did not analyze the method of administration of ADT due to heterogeneity of ADT administration and that might be influenced survival. Some of the PSA kinetics evaluated in this meta-analysis had significant high heterogeneity. The strengths of this study include (i) a high quality of study based on NOS scale; (ii) meta-analysis just included study with multivariate analysis (iii) several parameters that were associated with the survival were found in this study and might be evaluated in the future research.
In this study, the intial PSA before administering ADT did not influence the PFS or OS/CSS. Higher PSA nadir during ADT treatment was associated with shortened progression time and survival. A longer time to nadir is a good prognosis of progression and survival of mPCA treated with ADT.
Dataset 1. Quality assessment (based on NOS), hazard ratio, and standard error of studies included in initial PSA parameter. For quality assessment a maximum 7 stars could be scored; 6 or 7 stars considered as high quality study, 4 – 5 stars corresponded with intermediate quality, and 0 – 3 stars showed low quality. 10.5256/f1000research.14026.d19555334
Dataset 2. Quality assessment (based on NOS), hazard ratio, and standard error of studies included in PSA nadir parameter. For quality assessment a maximum 7 stars could be scored; 6 or 7 stars considered as high quality study, 4 – 5 stars corresponded with intermediate quality, and 0 – 3 stars showed low quality. 10.5256/f1000research.14026.d19557335
Dataset 3. Quality assessment (based on NOS), hazard ratio, and standard error of studies included in time to nadir parameter. For quality assessment a maximum 7 stars could be scored; 6 or 7 stars considered as high quality study, 4 – 5 stars corresponded with intermediate quality, and 0 – 3 stars showed low quality. 10.5256/f1000research.14026.d19557736
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Nayyar R, Sharma N, Gupta NP: Prognostic factors affecting progression and survival in metastatic prostate cancer.Urol Int. 2010; 84 (2): 159-63 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 28 Feb 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)