Keywords
acute respiratory distress syndrome, pneumonia, congestive heart failure, hypertension, left ventricle
acute respiratory distress syndrome, pneumonia, congestive heart failure, hypertension, left ventricle
Feedlot cattle are susceptible to two diseases of the cardiopulmonary system as they approach slaughter weight: congestive heart failure1 and acute interstitial pneumonia (AIP)2. In human medicine, and for the purpose of our study, acute interstitial pneumonia was defined as an acute respiratory distress syndrome (ARDS) of unknown etiology that is not primarily attributable to hydrostatic edema due to congestive left ventricular failure, pulmonary venous hypertension, or fluid overload3. In veterinary medicine, a consensus definition of ARDS has been proposed4. Diagnosing feedlot cattle using the proposed criteria, however, is not feasible largely because the diagnostic tools for ruling out hydrostatic edema are not available; consequently, it has been said that definitive diagnosis of AIP in cattle requires histopathologic evaluation of lung tissue obtained postmortem5. The histological counterpart of ARDS is typically diffuse alveolar damage (DAD), defined by the presence of hyaline membranes6.
If an animal dies in the acute, exudate phase of AIP, then hyaline membranes and hemorrhage may be the only lesions observed5. This is problematic because these lesions are not pathognomonic for ARDS and may be attributable to hydrostatic edema. Furthermore, there are two notable findings of clinically healthy feedlot cattle that suggest they may be at risk of congestive left heart failure, and therefore pulmonary hydrostatic edema, during the finishing phase: increased pulmonary arterial wedge pressures (PAWP) and pulmonary venous hypertrophy7. This leads us to speculate whether cattle presenting with clinical signs and pathology consistent with early stage AIP could, at least in some instances, be attributable to hydrostatic edema associated with left ventricular failure. The goal of this study was to determine if increased PAWP due to left ventricular dysfunction in a Holstein calf model could lead to pulmonary lesions consistent with the early, exudative phase of AIP in feedlot cattle.
Six, day-old male, intact clinically healthy Holstein dairy calves were collected from a farm in West Texas and individually housed under normoxic conditions (altitude: 975 m). At 7-days of age (Day 1 of the study), calves (n = 3) were given daily subcutaneous injections of the nonspecific ß-adrenergic agonist isoprenaline (10 mg/kg/d) (henceforth, ISO calves) or an equivalent volume of sterile water (controls, n = 3) for 14 days. On Day 14, pulmonary arterial pressures and wedge pressures were measured and echocardiography performed. Calves were euthanized on Day 15. The heart and lungs were weighed, and lung sections histologically evaluated.
Institutional Animal Care and Use Committee approval was granted prior to initiation of the study (Protocol 17013-02). Efforts were made to ameliorate animal suffering by following recommendations for the housing and care of animals provided by the National Research Council8.
Day-old male, intact, male Holstein calves were obtained from one commercial dairy in West Texas. Six calves were included in this preliminary study as, to our knowledge, no similar studies had been performed in this species. Calves were fed 4 to 5 L of colostrum within 12 hours of birth. They were given 3 L of non-medicated milk replacer (22% crude protein, 15% crude fat) twice per day throughout the study (Purina® Herd Maker®, Gray Summit, MO, USA) and had ad libitum access to water. From 1 week of age (Day 1) calves had ad libitum access to a calf starter (Purina®, Ampli-Calf® Starter 22, Gray Summit, MO, USA) with 22% crude protein (dry matter basis).
All calves were individually housed in pens with dimensions 1.8 m by 2.3 m (Agri-Plastics, Grassie, ON, Canada). Four calves were housed on a raised slatted floor inside temperature-controlled chambers (temperature 17 ± 3°C). Calves were housed according to body mass so that the heaviest calves at the start of the study (one control and one experimental calf) were housed in one chamber (Calves 4 and 5) and the lightest calves (Calves 1 and 2) in another chamber. One control (Calf 3) and one experimental calf (Calf 6), were housed in shaded outdoor pens with straw bedding on a sloped concrete floor. The pens were moved to new locations every 3 days and the inside of the pens cleaned with disinfectant (Virkon S, DuPont, Wilmington, DE, USA). Soiled straw was removed once daily, and all straw was replaced every 3 days.
To our knowledge, isoprenaline (DL-Isoproterenol hydrochloride, 98%, Alfa Aesar, Ward Hill, MA, USA) has not been used in large animal models of heart failure with preserved ejection fraction (HFpEF). The dosage used in this study (10 mg/kg sid) was double the dosage used in a study of equivalent duration in a mouse model9. The isoprenaline was dissolved in sterile water (50 mg/mL) at room temperature prior to subcutaneous injection in the neck. Alternating sides of the neck were used. Controls were injected subcutaneously in the neck with an equivalent volume of sterile water (0.2 mL/kg). Treatments were given between 8:30 am and 9:30 am after all calves had been fed milk replacer. Injections were given whilst calves were manually restrained within their pen.
Pulmonary arterial pressure (PAP) testing was performed on Day 14. Calves were restrained in a calf chute and a halter used to hold the calf’s head to one side so that the right jugular grove was exposed. The neck was clipped and cleaned with chlorhexidine solution. A 7-French peel-away introducer (IS-07AS, Vascor Medical Corporation, Tarpon Springs, FL, USA) was placed in the jugular vein prior to inserted of a 110 cm, 7 French, polyurethane, modified J-tip wedge pressure catheter (172-110P, Vascor Medical Corporation, Tarpon Springs, FL, USA). A pressure transducer (TranStar DPT, Smiths Medical ASD, Inc., Dublin, OH, USA) was interposed between the catheter to the data acquisition system (IX-TA-220, iWorx Systems, Inc., Dover, NH, USA). The pressure waveforms were recorded and analyzed offline (LabScribe3, iWorx Systems, Inc., Dover, NH, USA). Pressures were analyzed at the end of expiration. The position of the catheter tip was determined by monitoring the change in the pressure waveform as the catheter tip was advanced through the right atrium, right ventricle, and finally into the pulmonary artery.
Echocardiography was performed on Day 14. Calves remained standing throughout the procedure. Fractional shortening and ejection fraction (cubed or Teichholz method10) were obtained from right parasternal long-axis views of the left ventricle obtained between intercostal spaces 3 to 6. Relative wall thickness was calculated as two-times the left ventricular free wall diastolic thickness divided by the left ventricular internal diastolic diameter. Early diastolic (e’) septal lengthening velocities and early mitral flow (E) velocities were obtained from a left parasternal apical four-chamber view. The E/e’ ratio is a measure of left ventricular filling pressure and, consequently, a diagnostic measure of diastolic dysfunction11.
Calves were euthanized with intravenous pentobarbital sodium (85 mg/kg) on Day 15 of the study and exsanguinated. Lung lobes were individually weighed. The atria were separated from the ventricles at the atrioventricular junction. The right ventricular free wall (RV) was separated from the left ventricle and septum (LVS). The RV and LVS were individually weighed.
The left diaphragmatic lobe was perfused with formalin (10%, neutral buffered) at 15 to 20 cm H2O for approximately 5 minutes. After 5 days of formalin fixation, lung sections were collected midway along the dorsal aspect of the lobe for histology. Paraffinized sections (4 µm) were stained with hematoxylin and eosin and Masson’s trichrome. Lung sections were semi-quantitatively scored based on severity (0 = no lesion; 1 = mild; 2 = moderate; 3 = severe). The investigator was blinded to the calves’ identities and treatment group. The pulmonary parenchymal lesions scored included interstitial edema, intra-alveolar edema, hemorrhage and hemosiderin, inflammatory infiltrates, type II hyperplasia, and interstitial fibrosis. Pulmonary arterioles (< 500 μm) and veins were scored for medial hypertrophy and adventitial fibrosis. Veins were distinguishable from arteries as they have spiral bundles of muscle that give them a beaded appearance.
Statistical analyses were performed using commercial software (Stata version 12.1, College Station, TX). Calf 1 was excluded from statistical analyses because a sub-endocardial lesion was detected postmortem that likely affected his cardiac function and, consequently, pulmonary vascular pathophysiology. Between group differences were evaluated using Student’s t-test with equal variances. Student’s t-test is a suitable statistical method for small sample sizes (n ≤ 5) even if group sizes are unequal as long as the effect size is expected to be large12. Linear regression was used to determine if there was a significant difference between groups in the mass of the right ventricle, left ventricle and interventricular septum, and lung, while controlling for body mass.
Calves that received ISO had a greater mean PAWP but a lower mean PAP than controls. Mean PAWP was 12 ± 1 mm Hg in the ISO group and 7 ± 1 mm Hg in the control group (P = 0.01). Mean PAP was 23 ± 2 mm Hg in the ISO group and 43.5 ± 8 mm Hg in the control group (P = 0.05). The control group had a greater heart rate (104 ± 9 bpm) than the ISO group (74 ± 4 bpm; P = 0.03). Results are presented in Table 1.
| Group | Calf | Right ventricle | Pulmonary artery | Mean PAWP | Heart rate |
|---|---|---|---|---|---|
| Control | 1* | 51/-12 (19) | 30/11 (20) | 5 | 149 |
| 3 | 65/5 (35) | 68/40 (51) | 6 | 95 | |
| 5 | 68/-6 (30) | 48/15 (36) | 7 | 112 | |
| ß-AA | 2 | 42/1 (12) | 39/6 (20) | 10 | 71 |
| 4 | 43/3 (8) | 46/3 (22) | 13 | 70 | |
| 6 | 42/7 (16) | 43/19 (27) | 12 | 81 |
There was no statistical difference in ejection fraction or fractional shortening between the ISO and control groups indicating that left ventricular systolic function was preserved. Ejection fraction was 66 ± 6% in the control calves and 54 ± 6% in the ISO group (P = 0.29). Fractional shortening was 36 ± 4% in the control calves and 29 ± 4% in the ISO group (P = 0.27). ISO calves tended to have greater relative wall thickness than control calves (P = 0.15) and greater E/e’ ratios (P = 0.16) which is suggestive of concentric hypertrophy and diastolic dysfunction, respectively. Results are presented in Table 2.
| Group | Calf | Ejection fraction,% | Fractional shortening,% | Relative wall thickness | E/e’ |
|---|---|---|---|---|---|
| Control | 1* | 60 | 32 | 0.55 | 13.2 |
| 3 | 61 | 30 | 0.34 | 8.1 | |
| 5 | 60 | 32 | 0.37 | 8.3 | |
| ß-AA | 2 | 43 | 22 | 0.38 | 11.2 |
| 4 | 63 | 34 | 0.52 | 10.0 | |
| 6 | 57 | 30 | 0.49 | 8.7 |
There was no difference in body mass at the end of the study (P = 0.51). The mean body mass was 46.6 kg ± 3.3 kg for the control calves and 42.8 ± 3.4 kg for the ISO group. Calf 1 had endocardial fibrosis extending from the atrio-ventricular boundary to the dorsal aspect of the papillary muscle and approximately 0.5 cm into the myocardium (Supplementary file). Calf 5 had a 1 cm abscess in the right diaphragmatic lobe and Calf 6 had acute fibrinous pneumonia affecting the ventral 20% of the right cranio-ventral lung lobe (Supplementary file). The lungs of Calf 2 appeared hyper-inflated (Figure 1) and Calf 6 had a mottled icteric liver with rounded margins.
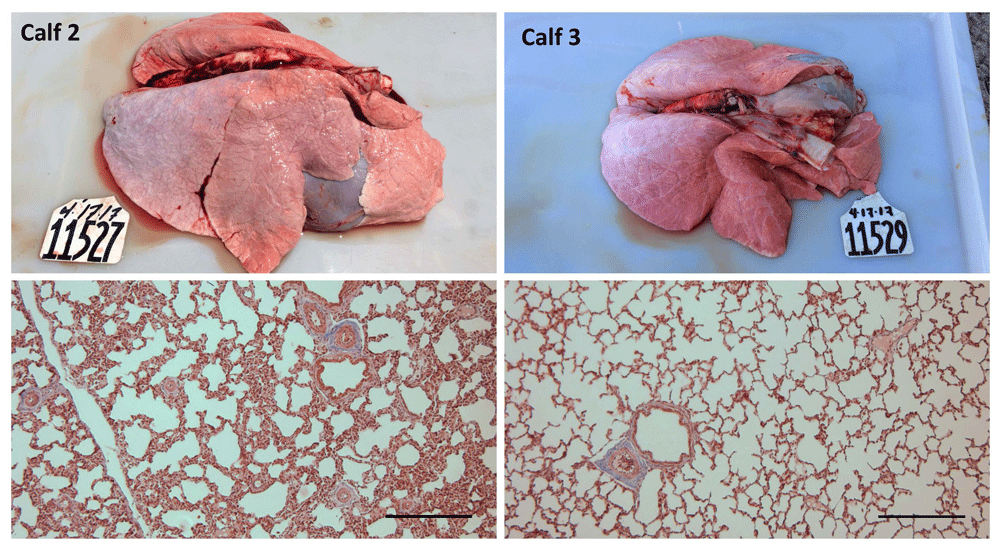
Masson’s trichrome. Scale bar = 0.25 mm.
The ISO group tended to have a left ventricle and interventricular septum that was 29 ± 10 g heavier than control calves (P = 0.10) when controlling for body mass. There was no difference between groups in the mass of the lung (P = 0.81) or right ventricle (P = 0.36) when controlling for body mass. Results are presented in Table 3.
| Group | Calf | Body mass, kg | Left ventricle and septum, g | Right ventricle, g | Lung, g |
|---|---|---|---|---|---|
| Control | 1* | 32.5 | 161 | 69 | 466 |
| 3 | 43.3 | 191 | 82 | 650 | |
| 5 | 49.8 | 208 | 83 | 797 | |
| ß-AA | 2 | 42.8 | 207 | 86 | 597 |
| 4 | 48.7 | 245 | 90 | 812 | |
| 6 | 37 | 174 | 74 | 556 |
In general, tissue areas with lesions consistent with DAD were multifocal and often located adjacent to a lobule with a normal healthy appearance. Occasionally, a gradual change from healthy to DAD was observed within the same lobule. All ISO calves had mild or moderate interstitial and intra-alveolar edema, mild or moderate hemorrhage, and type II hyperplasia. Two control calves (Calves 3 and 5) showed mild interstitial edema in some areas. Interstitial inflammatory infiltrates were evident in two calves, particularly Calf 1. No calves showed evidence of interstitial fibrosis. Calves 4 and 6 showed moderate epithelisation of alveoli. Calf 6 also had severe emphysema and honeycomb parenchymal remodeling (Figure 1). Results are presented in Table 4.
| Group | Calf | Interstitial edema | Hyaline membrane | Hemorrhage | Inflammatory infiltrates | Type II hyperplasia | Fibrosis |
|---|---|---|---|---|---|---|---|
| Control | 1* | 0 | 0 | 1 | 2 | 1 | 0 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 1 | 0 | 0 | 0 | 0 | 0 | |
| ß-AA | 2 | 2 | 2 | 2 | 1 | 2 | 0 |
| 4 | 2 | 2 | 2 | 0 | 2 | 0 | |
| 6 | 2 | 1 | 1 | 0 | 1 | 0 |
Mild or moderate medial hypertrophy and adventitial fibrosis of pulmonary arterioles was evident in all calves. ISO calves had more substantial medial hypertrophy of the pulmonary veins than controls. Pulmonary veins were not evident in Calf 6. Adventitial fibrosis was not observed except in Calf 4. Results are presented in Table 5.
| Pulmonary arteriole | Pulmonary vein | ||||
|---|---|---|---|---|---|
| Group | Calf | Medial hypertrophy | Adventitial fibrosis | Medial hypertrophy | Adventitial fibrosis |
| Control | 1* | 1 | 2 | 0 | 0 |
| 3 | 1 | 2 | 1 | 0 | |
| 5 | 2 | 1 | 1 | 0 | |
| ß-AA | 2 | 1 | 2 | 2 | 0 |
| 4 | 1 | 2 | 2 | 2 | |
| 6 | 1 | 1 | - | - | |
The findings of this study indicate that left ventricular dysfunction could feasibly contribute to the development of diffuse alveolar damage, the pathologic correlate of acute interstitial pneumonia, an ARDS of feedlot cattle of unknown etiology. Two weeks of daily injections with isoprenaline led to the development of heart failure with preserved ejection fraction (HFpEF); left ventricular systolic function was preserved but diastolic function was reduced. Given that subclinical HFpEF in a Holstein model has many of the pathologic features and physiologic measurements consistent with ARDS13, this preliminary study indicates that the diagnosis of AIP in a feedlot animal can only be considered as tentative if left ventricular dysfunction has not been ruled out as a primary cause.
The PAWP of the ISO calves were significantly greater than controls but substantially less than the PAWP of 26 mm Hg previously reported in feedlot cattle at the end of the feeding period at the altitude of 1,220 m7. Pulmonary arterial wedge pressures are typically less than 15 mm Hg in non-feedlot cattle and other mammals14; therefore, the DAD seen in the ISO calves in this study is likely attributable to the acute doubling of PAWP over a 2-week period. Feedlot cattle may be able to tolerate much greater PAWP if pressures were to rise insidiously throughout the feeding period. There are a variety of adaptations, in addition to pulmonary arterial and venous remodeling15, that may occur in response to rising pulmonary vascular pressures: first, the lymphatic system is recruitable – it is able to increase the rate of lung-water clearance by over 10-fold.16,17; and second, alveolar fibrosis may occur to reduce alveolar-capillary membrane permeability to water18. The time period over which pulmonary vascular pressure rise is, therefore, likely to be a key determinant of the pulmonary adaptability.
It is also feasible that isoprenaline induced DAD through forward hemodynamic effects. Increased cardiac contractility and pulse pressure may have had deleterious downstream effects on the pulmonary vasculature19,20 paving the way for increased fluid flux into the pulmonary interstitium. Whether attributable to backward or forward hemodynamic effects, an increase in pulmonary capillary pressure will lead to mechanical injury of the alveolar-capillary membrane leading to increased capillary permeability and impaired gas exchange21. Although acute damage can be reversed, long term blood-gas barrier disruption leads to lung fibrosis, inflammation, impaired alveolar fluid clearance, and muscularization of pulmonary vessels21,22.
Interestingly, the two calves housed in shaded outdoor pens on straw bedding had the greatest pulmonary arterial pressures within their respective treatment groups. In a prior study, calves housed on straw that developed bloody scours had histologic evidence of DAD and greater pulmonary arterial pressures than calves housed on slatted flooring that did not develop scours23. It was speculated that intestinal disease may contribute to the development of pulmonary disease in cattle23. Calves housed on straw bedding in our study may have been in the incubation phase of intestinal disease.
Even though there were only 6 calves in this study, the goal of the study, to determine if left ventricular dysfunction could lead to lesions consistent with the early, exudative phase of AIP, was met. For ethical reasons, decompensated heart failure was not induced; therefore, it is not possible to say whether overt left ventricular failure clinically manifests as an ARDS-like event. Human medical reports suggest, however, that it can present as an ARDS which is why the diagnosis of ARDS requires that acute dyspnea attributable to hydrostatic edema must first be ruled out3. Large-scale prospective cohort studies of feedlot cattle are necessary to determine if pulmonary arterial pressures and PAWP are positively associated with risk of respiratory diseases, such as AIP. We speculate that elevated pulmonary vascular hydrostatic pressures may act synergistically with mediators of acute alveolar damage, such as pathogens or airborne irritants, to promote the development and progression of respiratory disease.
All raw data underlying this study is available from Harvard Dataverse under a CC0 Public Domain Dedication
Images of the heart and lungs are available at http://dx.doi.org/10.7910/DVN/HD1GEI24
Pulmonary histology is available at http://dx.doi.org/10.7910/DVN/UR9MAC25
Hepatic histology images are available at http://dx.doi.org/10.7910/DVN/ZBIVAG26
Pulmonary vascular pressure recordings are available at http://dx.doi.org/10.7910/DVN/SKBMWZ27
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cattle model of hypoxia induced pulmonary hypertension, including pro-inflammatory phenotype and metabolic reprogramming of pulmonary vascular cells
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Lung disease of cattle, including lung infection, and immunity to lung disease caused by viruses or bacteria
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Mar 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)