Keywords
cytoglobin, neuroglobin, acetylcholinesterase, brain, intermittent hypobaric hypoxia
cytoglobin, neuroglobin, acetylcholinesterase, brain, intermittent hypobaric hypoxia
Cytoglobin (Cygb) and neuroglobin (Ngb) are globin proteins, found recently, which have special roles such as for oxygen supply in hypoxia. Both Cygb and Ngb have a high affinity for oxygen. These two globin proteins, which expressed by the neuron, are also known to act as scavengers of reactive oxygen species (ROS) and reactive nitrogen species1. Increase in Cygb and Ngb occurs in hemorrhagic stroke patients with increased ROS. Therefore, it is suggested that an increase in Cygb and Ngb are adaptation responses to increase oxygen supply and scavenge ROS due to hypoxia in hemorrhagic stroke2,3. Increased expression of Cygb also occurs after chronic systemic normobaric hypoxia induction in a rat's brain. An increased level of HIF-1α occurs along with the increased level of Cygb until day-7 of the induction, while the expression of Ngb was decreased in day 1, 3, 5, 7, and 14 of induction compared to normoxia4.
Decreasing the level of oxygen causes energy production depletion needed by the cells for their optimal physiological function, such as a cognitive function of the brain. The cholinergic system is known to form synapses throughout the brain, including the cerebral cortex, which is part of the cognitive signaling pathway. Cholinergic signaling is terminated, among other things, by the work of acetylcholinesterase (AChE), which catalyzes the hydrolysis of ACh in the synaptic cleft to an inactive form, choline and acetic acid5.
In systemic chronic normobaric hypoxia, there was an increase of AChE specific activity, which proves that the induction is able to trigger an increase of the enzyme activity, so that it can reduce the cholinergic system function6. In an animal study, bilateral common carotid artery occlusion caused chronic cerebral hypo perfusion. At week 4, the level of ACh in the hippocampus decreased. ACh reduction also occurred in the striatal area, and was suspected to be the cause of memory consolidation disturbance that leads to learning dysfunction7.
Mulyawan's research has shown that HIF-1α expression was increased after intermittent hypobaric hypoxia induction in a rat using hypobaric chamber8. Until now, there is no data of how IHH affects Cygb and Ngb expression, which play a role in oxygen supply, and how this affects AChE specific activity, which plays a role in ACh, a neurotransmitter important for cognitive function, termination. The purpose of this study is to determine the effect of IHH toward these three parameters in the brain adult male of Sprague-Dawley rats.
All procedures were approved by the Ethics Committee of the Faculty of Medicine Universitas Indonesia Rumah Sakit Cipto Mangunkusumo No. 626/UN2.F1/ETIK/2014. All efforts were made to ameliorate any suffering of animals used in this research. The rats were observed daily to confirm their healthy condition. The behavior tests, done by Farhan[18], and the hypoxia induction did not involve painful stimuli.
Based on Federer’s rule, with five groups of induction, twenty-five adult Sprague-Dawley male rats from Badan Pengawasan Obat dan Makanan Republik Indonesia (BPOM RI), initially weighing 200–250 grams, were randomly divided into 5 groups: 1) The control group (normoxia); 2) group exposed to acute hypobaric hypoxia (AHH, control to intermittent hypobaric hypoxia (HH) treatment); 3) group exposed to HH on day-1 and re-exposed on day-8 (intermittent hypobaric hypoxia once, IHH1x); 4) group exposed to HH on day-1, re-exposed to HH on day-8 and day-15 (intermittent hypobaric hypoxia 2 times, IHH2x); 5) group exposed to HH on day-1, re-exposed to HH on day-8, day-15 and day-22 (intermittent hypobaric hypoxia three times, IHH3x).
Before the experiment all rats were kept under optimal conditions: 12:12 hours light to dark cycle at 24°C, in the animal house of Indonesian Air Force Institute of Aviation Medicine, LAKESPRA dr. Saryanto, Jakarta, with food and water ad libitum. Pelleted food and tap water were given. Wire cages were used to ensure optimum ventilation; subjects in a group were put in the same cage. Shredded newspaper were used and changed daily.
After treatment, rats were euthanized by cervical dislocation. Then the brains were immediately removed, weighed and divided into aliquots and stored in -80°C.
The procedure of hypobaric hypoxia is referred to hypobaric hypoxia type I chamber flight profile, modified by Mulyawan, presented in Figure 18.
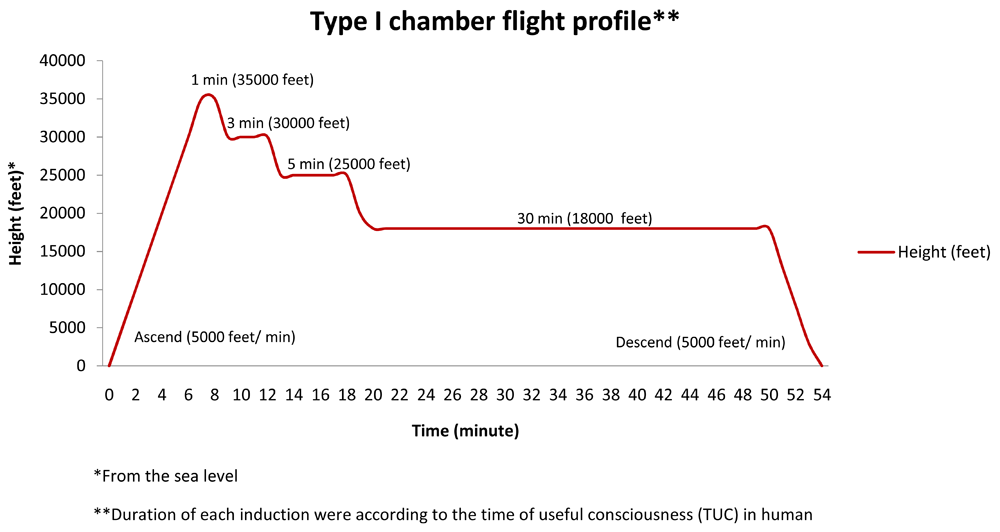
Produced and previously presented by the first author at The 1st Asian Researcher Symposium at the Universitas Indonesia-Depok, April 26th 2016.
In this procedure, starting around 09.00 AM, the rats were put in a hypobaric chamber at the Aerophysiology Department of Indonesian Air Force Institute, LAKESPRA dr. Saryanto, Jakarta, and exposed to treatment ascending with rising speed of 5,000 feet/ minute until reach the altitude which equal to 35,000 feet above sea level for 1 minute, then descending gradually to 30,000; 25,000; 18,000 feet for 3; 5; and 30 minutes respectively. And then descend gradually until reaching the sea level. All descending speed is performed at 5,000 feet/ minute8.
Brain tissue was homogenized using RIPA Lysis Buffer (Santa Cruz ®, product number sc-24948) before the measurement of the total protein, Cygb and Ngb proteins, using ELISA method and specific activity of AChE. These activities were conducted at the Laboratory of Molecular Biology for Oxidative Stress Studies, Department of Biochemistry and Molecular Biology, Faculty of Medicine Universitas Indonesia, Salemba, Jakarta.
Total protein concentration is determined using bovine serum albumin (BSA) as standard solution (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 mg/dL) and absorbance was read at λ 280 nm using Nano drop Varioskan Flash (Thermo Scientific).
Cygb protein concentration was measured using ELISA method. The microplate provided in the kit has been pre-coated with Cygb specific antibody. The standard solutions of Cygb were made as follows: 0 (blank); 0.78; 1.56; 3.12; 6.25; 12.5; 25; and 50 ng/mL. The absorbance was read at λ 450 nm. The concentration of Cygb was measured using Cygb standard curve. The concentration of Cygb in brain tissue was presented as the concentration of Cygb/total brain protein concentration (ng/mg protein).
Ngb protein concentration is determined using ELISA method. Ngb specific antibody has been pre-coated onto the microplate. The standard solutions of Ngb were made as follows: 0 (blank); 15.6, 31.2, 62.5, 125, 250, 500, and 1000 ng/dL. The absorbance was read at λ 450 nm. The concentration of Ngb was measured using Ngb standard curve. The concentration of Ngb in brain tissue was presented as the concentration of Ngb/total brain protein concentration (ng/mg protein).
In this assay, thiocholine as the product of AChE is reacted to 5,5'-dithiobis (2-nitrobenzoic acid, DTNB) to form a yellowish color product, which provides absorption at λ 412 nm. The intensity of the yellowish color is equivalent to the activity of AChE. One unit of AChE is a number of enzymes that catalyze the production of 1.0 µmol tiocholine/minute at pH 7.5 at room temperature. The specific activity of AChE was measured by dividing the AChE activity with the total protein of each sample (U/mg protein).
Graph Pad Prism version 7.0 was used for statistical analysis. Data normality was examined using D’Agostino and Pearson normality test. Then data were analyzed using ANOVA for significance and further post hoc with Tukey test. The Pearson correlation test was used to examine the correlation between parameters.
The concentration of Cygb in brain tissue after induction of AHH, IHH1x, IHH2x, IHH3x, and normoxic groups, is shown in Figure-2. In this figure, we can see that there were significant differences between the IHH2x and IHH3x groups compared to normoxic group (p=0.015 and p=0.000, respectively). There was also a significant difference between IHH1x, IHH2x and IHH3x compared to the AHH group (p=0.02, p=0.001 and p=0.000, respectively). Differences also appeared between IHH1x and IHH3x (p = 0.005).
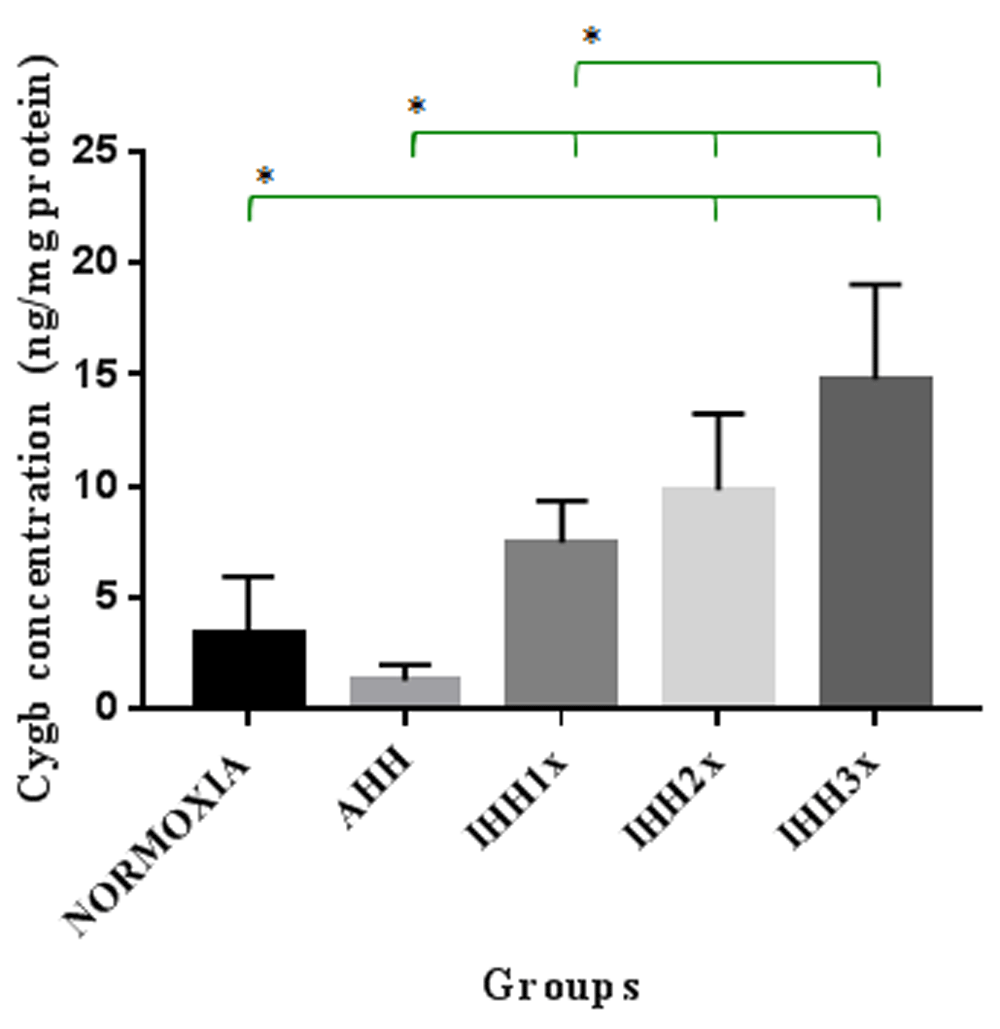
AHH, control to intermittent hypobaric hypoxia treatment; IHH1x, intermittent hypobaric hypoxia once; IHH2x, intermittent hypobaric hypoxia 2 times; IHH3x, intermittent hypobaric hypoxia three times. *p<0.05.
It is shown in Figure-3 that there are significant differences between the IHH2x and IHH3x groups compared to normoxic group (p=0.049 and p=0.03, respectively). There were also significant differences between IHH2x and IHH3x groups compared to the AHH group (p = 0.005 and p=0.000, respectively). Differences were also showed between IHH2x and IHH3x groups compared to IHH1x group (p = 0.022 and p=0.001, respectively).

AHH, control to intermittent hypobaric hypoxia treatment; IHH1x, intermittent hypobaric hypoxia once; IHH2x, intermittent hypobaric hypoxia 2 times; IHH3x, intermittent hypobaric hypoxia three times. *p<0.05.
As can be seen in Figure 4, there were differences in the specific activity of AChE in brain tissue of rats in IHH2x and IHH3x groups compared to normoxic group (p=0.000 and p=0.000, respectively). There were also significant differences between IHH2x and IHH3x with the AHH group (p=0.000 and p=0.000, respectively). There were also significant differences between the IHH2x and IHH3x groups compared to IHH1x group (p=0.000 and p=0.000, respectively), and between IHH2x and IHH3x (p=0.026).
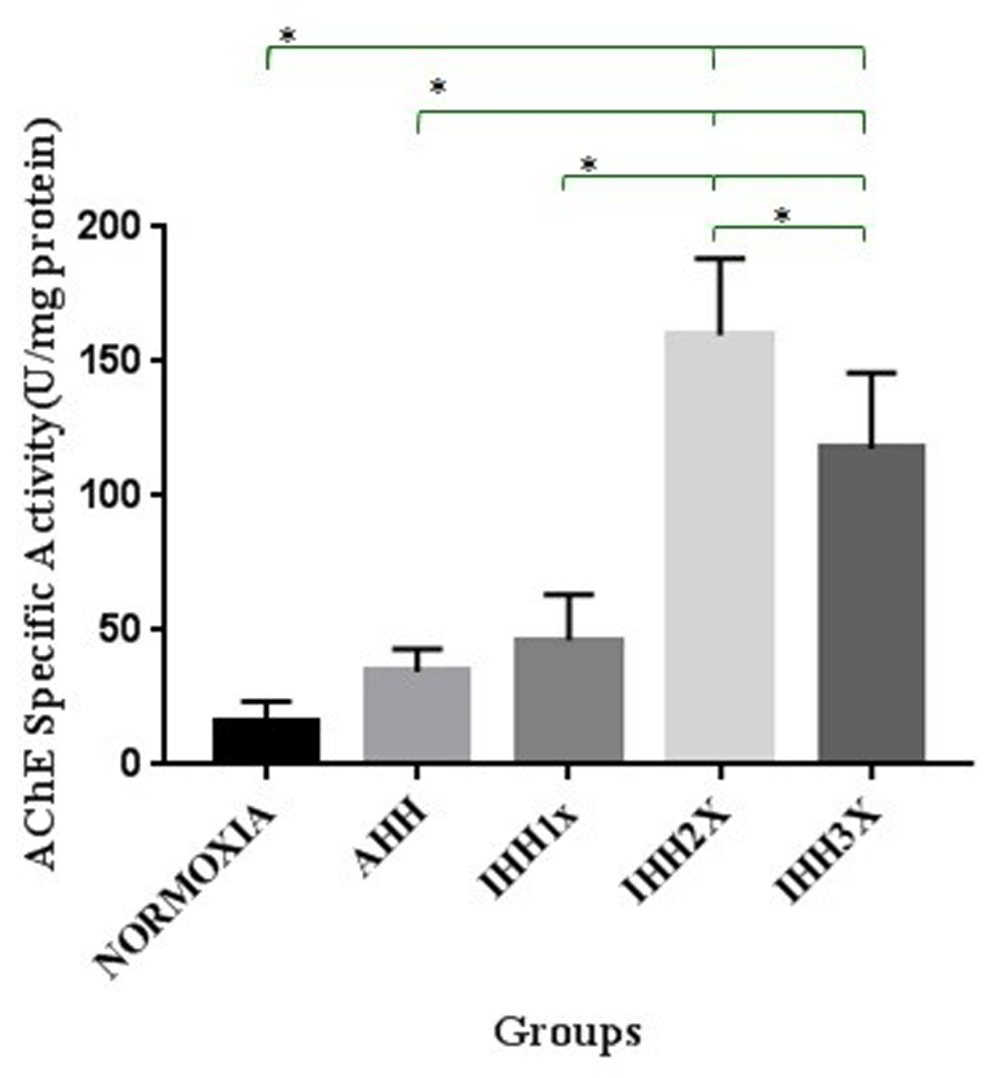
AHH, control to intermittent hypobaric hypoxia treatment; IHH1x, intermittent hypobaric hypoxia once; IHH2x, intermittent hypobaric hypoxia 2 times; IHH3x, intermittent hypobaric hypoxia three times. *p<0.05.
There was a strong positive correlation between the Cygb protein and specific activity of AChE in brain tissue of normoxic, AHH and IHH groups (Pearson, r = 0.728 and p<0.0001) (Figure-5a).
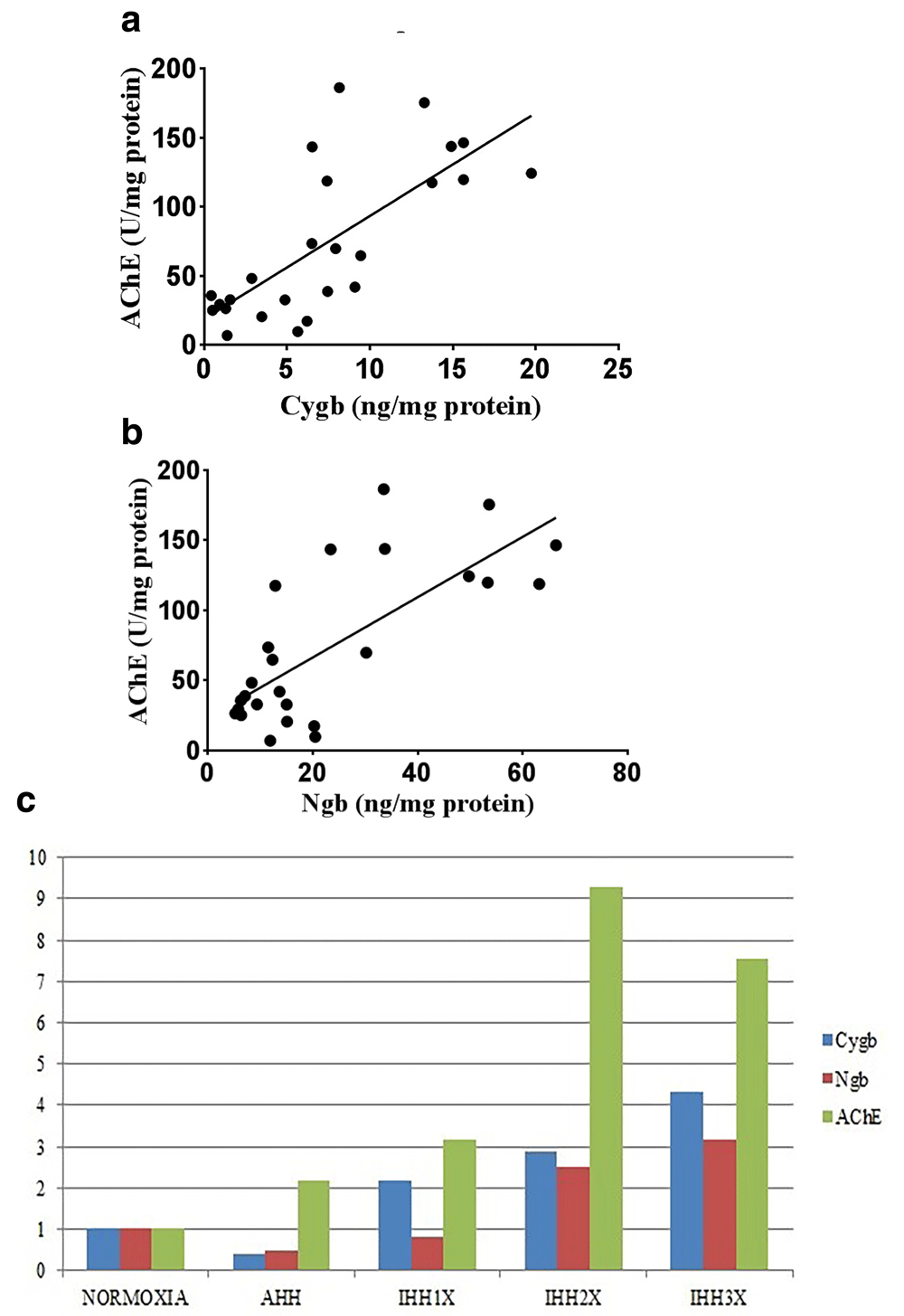
(a) Correlation between Cygb protein and AChE specific activity in brain tissue of rats (Pearson; r=0.728; p<0.0001). (b) Correlation between Ngb protein AChE specific activity in brain tissue of rats (Pearson; r=0.542; p<0.0001). (c) Comparison of Cygb, Ngb protein and specific activity of AChE in brain tissue of rats. AHH, control to intermittent hypobaric hypoxia treatment; IHH1x, intermittent hypobaric hypoxia once; IHH2x, intermittent hypobaric hypoxia 2 times; IHH3x, intermittent hypobaric hypoxia three times.
There was a moderate positive correlation between the Ngb protein and specific activity of AChE in brain tissue of normoxic, AHH and IHH groups (Pearson, r = 0.542 and p<0.0001) (Figure-5b).
Comparison of the expression patterns between Cygb, Ngb levels and the specific activity of AChE is shown in Figure-5c. It appears that Cygb and Ngb expressions were decreased while the specific activity of AChE was increased at AHH. At the induction of IHH1x, Cygb and Ngb expressions were increased, so did the specific activity of AChE. Induction of IHH2x caused an increase in Ngb and Cygb levels and also the specific activity of AChE. At the induction of IHH3x, Cygb and Ngb expressions were increased, however, the specific activity of AChE was decreased.
In this research, Cygb and Ngb expressions of AHH group were decreased compared to normoxia. Those two protein expressions were then increased in intermittent induction of hypoxia, from IHH1x induction until IHH3x. The low expressions of Cygb and Ngb in AHH group might be due to the role of these proteins in scavenging ROS produced in this induction. In repeated hypobaric hypoxia induction (IHH1x, IHH2x, and IHH3x), the increase of Cygb and Ngb expressions might be due to adaptation response toward hypoxia and the increased ROS in this condition. Cygb and Ngb are known to be increased in supplying oxygen and reducing or scavenging ROS in hypoxic conditions9–11.
The increase of specific activity of AChE was seen from AHH induction and continued to increase until IHH2x and then decreased in IHH3x. The increased specific activity of this enzyme was also reported by Muthuraju et al. in hypobaric hypoxia and Andriani et al. in chronic normobaric hypoxia6,11. An increase in AChE activity, which then caused the decrease of ACh concentration, in and around amyloid β-peptide (Aβ) (a substance which found to be increased in Alzheimer’s disease) has also been shown12. The increased activity of AChE which causes the decrease amount of ACh eventually caused a reduction in blood flow. The reduction of blood flow due to the decreased levels of ACh is due to the function of ACh in causing vasodilatation13. In brain hypoperfusion by ligating common carotid artery, there is a decrease in ACh in the hippocampal area, which also related to memory and learning impairment7. In addition, the loss of perivascular cholinergic terminals was shown in AD patients compared to aged controls14. This research was supported by other researchers who observed impaired cortical cerebral blood flow in patients with AD15.
Moreover, AChE inhibitor medication is known to affect cholinergic function in subjects treated with hypobaric hypoxia (decrease acetylcholinesterase activity, increase acetylcholine levels and upregulation of choline acetyltransferase—an enzyme that has a role in acetylcholine formation) and eventually memory function11. Inhibitors of AChE are also known to improve cerebrovascular function16. This medication is known to overcome the cognitive function impairment in Alzheimer’s disease17. As a comparison in cognitive function, Farhan reports that IHH, using the same procedure with our study, increases cognitive function compared to control group18.
In this research, the specific activity of AChE was increased in AHH conditions, which meant that there was a decrease in blood flow to the brain due to the decrease of ACh. This condition may lead to a decrease in blood flow, inducing the adaptation response through increased expressions of Cygb and Ngb since IHH1x until IHH3x to increase blood/oxygen supply. However, since the blood flow was not measured in this research, this limitation needs further research to ensure whether this hypothesis is correct. Meanwhile, in IHH3x there was a decrease in AChE specific activity. It suggests that in IHH3x the blood/oxygen flow is sufficient due to the increase expressions of Cygb and Ngb which cause an increase in oxygen availability. This phenomenon is supported by Mulyawan’s research that reports an increase in microvascular density using GLUT-1 as the marker from IHH1x until IHH3x induction8. However, the mechanism of the increase of AChE activity in hypoxia requires further study.
The present research has demonstrated that intermittent hypoxia (IHH1x until IHH3x) can provide protection against hypoxia, which was shown in the increase of Cygb and Ngb that supply oxygen. Overall, this research could be implemented for individual training to adapt to hypoxia conditions, for example, air force pilot.
We conclude that IHH seems to induce a protective adaptive response in the rat brain tissue through the changes of Cygb and Ngb expression and the changes of AChE specific activity. Further research is needed to measure and evaluate the blood flow changes and the ACh level and choline-acetyltransferase (ChAT), an enzyme that catalyzes ACh synthesis, using the same research model.
Dataset 1: Raw data for cytoglobin, neuroglobin and acetylcholinesterase specific activity measurements 10.5256/f1000research.13592.d19700619
This research was supported by grants from Hibah PITTA 2006-DRPM Universitas Indonesia and supported by Indonesian Air Force Institute, LAKESPRA dr. Saryanto, Jakarta.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Wardaya and his team (from Aerophysiology Department of Aindonesian Air Force Institute, LAKESPRA dr. Saryanto, Jakarta) for helping the induction of hypobaric hypoxia, Ondi Sutisna and Arif Nurdianto (from Molecular Laboratory for Oxidative Stress Studies, Department of Biochemistry & Molecular Biology, Faculty of Medicine Universitas Indonesia) for some reagents preparation, and dr. Shanty Olivia Febriyanti Jasirwan, Sp.OG from the Writing Center FKUI helping proofread this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Costa DC, Alva N, Trigueros L, Gamez A, et al.: Intermittent hypobaric hypoxia induces neuroprotection in kainate-induced oxidative stress in rats.J Mol Neurosci. 2013; 50 (3): 402-10 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Intermittent hypobaric hipoxia, exercisephysiology, altitude acclimatization
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hypoxia/ischemia, hypobaric hypoxia, brain, neuroprotection, cerebral mechanisms of hypoxic tolerance, expression of genes and proteins, neurochemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 04 Apr 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)