Keywords
B cell receptor, B cell differentiation, B cell development,
B cell receptor, B cell differentiation, B cell development,
B cells, the antibody-producing cells, have a protagonist role in the immune response. Although the presence and relevance of antibodies were established more than 100 years ago1 and antibody-producing cells were identified in the mid-20th century2, it took until 1965 for the distinctive B-cell lineage to be recognized3,4. Today, there are still many questions about B-cell differentiation during development and after activation and about the signals that govern such differentiation.
B cells undergo a diversification process during their development in bone marrow and fetal liver, and, as part of this differentiation, B cells rearrange the immunoglobulin (Ig) heavy (H) and light (L) chain gene loci to create a complete Ig molecule5,6. The commitment of the common lymphoid progenitor to the B-cell lineage can be recognized by the expression of the B220 isoform of CD456–8. B cells then develop through several well-characterized stages, ending with the expression of surface IgM and IgD class Ig molecules, which, in association with Igα and Igβ, form the B-cell receptor (BCR) for the antigen5,6. BCR signaling is required for B-cell maturation and survival, and BCR must provide tonic signals, either spontaneously or on interaction with ligands in the environment9–12. After this, B cells emerge to recirculate through secondary lymphoid organs such as the spleen and lymph nodes13. Being well positioned in the secondary lymphoid organs, mature naïve B cells are ready to respond to antigens. Recognizing the antigen through the BCR, B cells are activated and differentiate into plasma cells through extra-follicular differentiation14, or they become germinal center (GC) precursor cells to start GC reactions15,16. The precise signaling mechanisms of B-cell fate decision during this stage are not entirely understood.
In this review, we will focus on B-cell subsets in the spleen, key steps of differentiation after activation, and, in particular, recent findings about the role of the BCR driving these distinct differentiation stages (Figure 1).
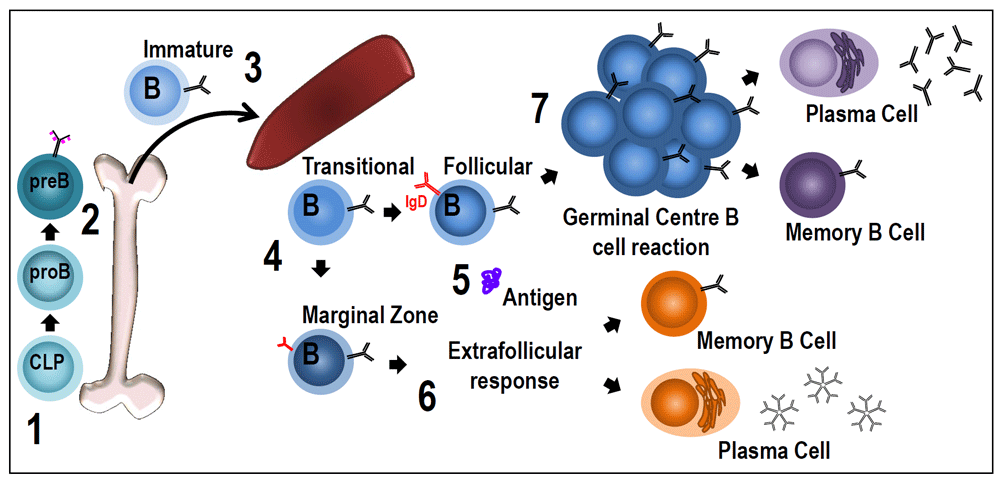
(1) Common lymphoid progenitor (CLP) cells commit to the B-cell lineage when they start expressing the B220 isoform of CD45. (2) Pro-B cells undergo DJ rearrangements and become pre-B cells when they express membrane forms of μ heavy chains with surrogate light chains (pink dotted lines) in the pre-B receptor. The transition between pro-B and pre-B cells has been described as being dependent on membrane association of Igα/Igβ complexes and their ability to generate basal signals. It seems that pre-B receptors do not have a ligand-recognition function40,41. (3) After VDJ recombination in the pre-B cell stage, immature B cells pair light chains with μ chains to form monomeric IgM, which is expressed at the cell surface in association with Igα/Igβ to form the B-cell receptor (BCR). Newly formed immature B cells exit the bone marrow to reach the spleen, where, as transitional B cells, they will complete their maturation before entering the follicles or the marginal zone (MZ). (4) BCR signaling strength appears to have a critical role during follicular (Fo) or MZ B-cell differentiation. There is evidence of at least two possibilities29,30,36,42–44: increased BCR signaling could drive differentiation to Fo B cells or to MZ B cells. This could depend on other factors such as the microenvironment or the timing of BCR signaling. (5) Mature naïve B cells are ready to respond to antigens. By specifically binding antigen through the BCR, B cells are activated and differentiate into plasma cells through extra-follicular differentiation (6) or start the T-cell-dependent germinal center (GC) reaction (7). The mechanism of activated B cells entering the GC reaction or undergoing rapid plasma cell differentiation in extra-follicular proliferative foci is controlled by the nature of the interaction between the BCR and antigen. Responding clones that undergo a strong initial interaction with antigen can efficiently differentiate into extra-follicular plasma cells45. However, it has also been shown that B cells expressing higher-affinity BCRs are more competitive to become pre-GC B cells during T–B interaction46,47. This seems contradictory, but timing may be a very important factor. B cells in the GC reaction are selected on the basis of their interaction with the antigen through BCRs. Based on how efficiently B cells present antigen to Fo T helper cells, they are allowed to further differentiate inside the GC. Whether this BCR–antigen interaction results in significant signaling and has a role for selection is not clear.
BCR signaling has been intensely studied over the last 30 years, and details of this complex process are still not fully understood. In-depth reviews have been produced by others17–20. In brief, BCR is activated by binding of the antigen. This leads to phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) by the first kinase in the BCR signaling pathway, primarily LYN (part of the src-kinase family). After this, SYK is recruited through its SH2 domain to the phosphorylated Igα–Igβ heterodimer. The higher propensity of ligand-bound BCR molecules to aggregate can enhance their association with src-family PTKs17,20. Once SYK is activated, the BCR signal is propagated via a group of proteins associated with the adaptor protein B-cell linker (BLNK, SLP-65). Phospho-BLNK serves as a scaffold for the assembly of the other components, including Bruton’s tyrosine kinase (BTK), VAV 1, and phospholipase C-gamma 2 (PLCγ2)19. The initiation of the BCR signal is indirectly regulated by at least two non-receptor-associated molecules: B220 and C-terminal src tyrosine kinase (CSK)17.
Additionally, following BCR ligation, tyrosines of the cytoplasmic tail of CD19 are phosphorylated by LYN to create binding sites for the SH2 domains of the p85 adaptor subunit of PI-3K as well as other SH2 domain-containing effectors. Activated PLCγ2 cleaves membrane-associated phosphoinositide PI(4,5)P2 into the second messengers I(1,4,5)P3 and DAG. I(1,4,5)P3 generation causes the mobilization of Ca2+ from intracellular and extracellular stores. Ca2+ signaling is required for the activation of transcription factors such as nuclear factor kappa B (NF-κB) and nuclear factor of activated T cells (N-FAT) by protein kinase C (PKC)21. DAG represents a classic activator of PKC isotypes which regulate the mitogen-activated protein kinase (MAPK) family (extracellular signal-regulated kinase [ERK], c-Jun NH2-terminal kinase [JNK/SAPK], and p38 MAPK); the overall result of these processes drives activation of the B cell, antigen presentation, cytokine production, and cell proliferation and differentiation17–19. In the following, we will discuss the effects of BCR signaling during B-cell development and after the encounter with the antigen.
Three major populations of mature B cells have been described in mice: B1, marginal zone (MZ), and follicular (Fo) B cells. The phenotypic, microanatomic localization and functional differences that characterize them suggest specialized functions linked to the niches in which they reside5. B1 cells produce polyreactive natural antibodies (NAbs) of relatively low affinity and primarily of the IgM isotype22. NAbs play a critical role in the innate immune response against pathogens, and their absence can lead to increased susceptibility to microbial infections23–25. B1 cells are the major subpopulation in pleural and peritoneal cavities; however, they can also be found in the spleen and other lymphoid organs but at low frequency26. B1 cells consist of two functional specialized subpopulations regarding the expression of CD5: CD5+ B1a and CD5− B1b cells27. However, expression of Blimp-1 also delineates two main coexisting B1 subpopulations in the bone marrow and spleen: B1 Blimp-1hi cells that are dependent on Blimp-1 for maximal natural Ig production and those B1 cells that neither express Blimp-1 nor require it for steady-state antibody production28. Recently, it has been shown that interleukin 17A (IL-17A) promotes B1-cell infiltration into lungs during viral infection, where B1a cells differentiate into IgM-producing plasma cells. This process was promoted through Blimp-1 expression and NF-κB activation25. It is conceivable that the regulation of Blimp-1 expression would also drive the functional role of B1 subsets in sites such as the lung.
What is the role of BCR signaling in B1-cell differentiation? Studies with genetically modified mice indicate that the strength of BCR signaling may control the development or persistence of B cells (or both)29–36. Mutations that enhance BCR signaling strength through the specific deletion of SHP-1 in B cells expand the B1a population. SHP-1 is a protein-tyrosine phosphatase expressed in hematopoietic cells and plays a signal-attenuating role in pathways initiated by many ITAM-containing receptors37–39. The signal-attenuating effects of SHP-1 are mediated primarily via its binding to inhibitory receptors, such as CD5, expressed by B1a cells34. Additionally, enhanced tonic BCR signaling results in an increased B1 B-cell subpopulation and a dysregulated homeostasis of other B-cell subsets33. These findings indicate that BCR signaling is important in fate decisions during B1 cell development, and further studies are needed to better understand these mechanisms.
MZ B cells contribute about 5–10% of the B cells in the spleen. The “marginal zone” designation refers to the splenic structure that separates the red and the white pulp adjacent to the marginal sinus, where—in both mice and humans—these MZ B cells are in direct contact with blood and its contents5,48. The specialized localization and migration of B cells are strictly regulated under the guidance of different chemokine–chemokine receptor pairs, such as CXCL13–CXCR5, S1PR1, and S1P49–53.
Blood from the primary sinusoids in the spleen perfuses the MZ; the anatomic location of MZ B cells facilitates their role as a rapid first line of defense against blood-borne particulate antigens52,54. After MZ B cells capture the antigen, they transport it to the follicles and deliver it to follicular dendritic cells (FDCs)52,53,55. Furthermore, MZ B cells respond to thymus-independent type 2 antigens producing high quantities of IgM and IgG314,56,57.
Newly formed B cells exiting the bone marrow reach the spleen at a relatively immature stage; these are termed transitional B cells and they need to complete their maturation in the spleen before entering the follicles or the MZ58. It has been described that B cells in the transitional 2 (T2) stage face a decision to mature into either Fo or MZ B cells5,48. However, very recently, it was shown that T1 B cells can differentiate to MZ B cells32. During this differentiation, signaling through the BCR is important for the Taok3-mediated acquisition of membrane expression of ADAM10, which cleaves Notch2 and CD2331,32. MZ B-cell instruction requires triggering of Notch2 on developing B cells by Delta-like 1 (Dll1) expressed by splenic red pulp sinus endothelial cells or MZ reticular cells35,59. How exactly B-cell-positive selection and BCR signaling are causing Taok3 activation and ADAM10 surface expression will require further study32. Furthermore, BCR signaling strength appears to have a critical role during MZ B-cell development; mice lacking secreted IgM displaying increased BCR signaling had increased MZ and decreased Fo B-cell numbers36,42,43. However, some studies reported that MZ B cells need low BCR signaling strength for their differentiation but that transitional B cells with higher BCR signaling strength favor differentiation into Fo B cells29,30,44.
Fo B cells are the most prevalent of the three subsets of B cells and the better-studied subpopulation. Their anatomic enrichment in primary follicles gives them their name; however, they are not confined to the follicles and also predominate among the mature populations of B cells in the bone marrow, blood, and other lymphoid organs5. Fo B cells are involved mainly in interactions with T cells, and their responses to T-cell-dependent antigens eventually originate GC reactions16. Many of the mechanisms producing the selection of B-cell subsets, the roles of self- and environmental antigens, and survival signals that drive or maintain them in their proper anatomic and functional niches remain to be elucidated. BCR signaling has been proposed to be crucial in the selection of B1, MZ, and Fo B cells, supported by different genetically manipulated mice where the altered BCR signaling affected different B-cell subsets32–34,36.
After Fo B cells encounter the antigen through the BCR, CXCL13–CXCR5 slows down the motility of B cells by promoting membrane ruffling and LFA-1-supported adhesion to facilitate the antigen-recognizing process and enhance B-cell activation60. Meanwhile, surface expression levels of CCR7 on responding B cells increase rapidly to make them more sensitive to CCL19/CCL2151. CCL19/CCL21 is expressed by T-zone reticular cells and extends a gradient to the Fo region. Along the chemokine gradient, antigen-engaged B cells migrate from follicles to T–B border in order to get signals from primed T cells51,61,62. EBI2 drives B cells to move back to the outer follicle and inter-follicular regions63,64. These results indicate interplay between activated BCR downstream signaling and surface chemokine receptors60,61. Although the possibility is less studied on steady state, tonic BCR signaling could also regulate chemokine receptor expression during their anatomic enrichment in specific areas within the secondary lymphoid organs.
After T–B cell interaction, activated B cells either differentiate into plasma cells in the extra-follicular response14 or become GC precursor cells, migrating back into follicles to start GC reactions16. To get co-stimulation from T cells, B cells need to present cognate antigens to T cells in the major histocompatibility complex II (MHC-II) context. B-cell-captured antigen goes through both extracellular and intracellular degradation to become peptides65. These peptides then are assembled with MHC-II molecules and expressed on the B-cell surface as peptide–MHC (pMHC). pMHC–T-cell receptor recognition has an essential role in the process of T–B “pairing” to ensure the specificity of later reactions. Schwickert et al. showed that a higher amount of pMHC help activated B cells, locking T-cell help on the T–B border, thus enabling them to become GC precursor B cells46. CD40L is the most important cognate signal delivered from T cells. In mice and humans, CD40–CD40L ligation is indispensable for the initial formation of GCs66,67 and is needed to maintain ongoing GC reactions68.
BCR affinity also plays an important role during the initial GC B-cell fate decision. The mechanism of activated B cells entering the GC reaction or undergoing rapid plasma cell differentiation in extra-follicular proliferative foci is controlled by the nature of the interaction between the BCR and antigen. Responding clones that undergo a strong initial interaction with antigen can efficiently differentiate into extra-follicular plasma cells and contribute to the rapid early thymus-dependentantibody response45. Although the requirements for GC entry are not stringent69, responding B cells expressing higher-affinity BCRs on their surface are more competitive to become pre-GC B cells during their T–B interaction46,47. High-affinity BCR captures more soluble antigens70 and leads to a higher amount of pMHC expressed on the B-cell surface, which results in a competitive cognate interaction with T cells. The durations of T–B interactions have critical roles in B-cell fate decisions. Recent research shows that ICAM-1/2 adhesion molecules on B cells can secure long-lasting T–B interactions to enhance T-cell help. The expression of ICAM-1/2 could compensate the lack of MHC-II signaling to form GC B cells71.
BCRs of B cells differentiating in GCs have to interact with antigen repetitively. Whether the BCR-antigen interaction in the GC results in significant signaling and what the role of this is are under debate72–74. The interaction is certainly important to test BCR specificity and binding competitiveness, probably mainly in competition with antibodies present in immune complexes on the FDC networks75,76. More important than BCR signaling may be that the affinity of the BCR–antigen interaction will let B cells take up more or less antigen, resulting in more or less efficient positive selection by Fo T helper cells47,74,76.
Much of the knowledge on B-cell differentiation in response to antigen has been gained by using BCR knock-in animals specific for haptens or single epitopes on model proteins46,77. Recent attempts to use complex protein antigens such as influenza hemagglutinin should be better suited to understand the complex competition and crosstalk of many different clones interacting with different epitopes on a natural complex protein antigen78,79. However, for such experiments, it is important to develop methods that will allow the specific analysis of epitope-specific interactions of different B-cell clones. Without an understanding of whether clonal interactions happen because of BCR binding of overlapping epitopes or whether clones develop with less competition because they do bind different epitopes, it is impossible to conclude whether clones with different affinities compete for the antigen80.
Ultimately, it has been estimated that nearly 50% of newly produced auto-reactive B cells can avoid deletion and are induced into an anergic state in peripheral lymphoid tissues81,82. Thus, perhaps B cells in the periphery are not in the same basal state as we thought they should be. The surface expression level of membrane IgM is downregulated on anergic B cells83,84, indicating that their BCR signaling is somehow different from that of normal naïve B cells. Indeed, anergic B cells maintain a distinct gene expression profile81,84 and have been observed preferentially residing in T-zone areas85, indicating that their surface chemokine receptor expression pattern is also different from that of naïve B cells, possibly because of their different signaling. Several studies have found that anergic B cells can be selected to become GC B cells, although these cells previously were thought to be non-responsive84,86. By recruiting anergic B cells into the GC response involving many rounds of mutation, these B cells may mutate away from their original auto-reactivity and become specific for antigens that may have a close relationship with autoantigens86–89. The mechanism—for example, whether anergic and normal B cells will follow the same rules of B-cell selection or which difference an anergic state can bring to B-cell activation and fate decisions at the T–B border—is still not clear.
Co-stimulation from T cells plays indispensable roles in B-cell fate decisions after activation and has been studied in some detail. However, BCR signaling strength and patterns not only affect B-cell selection during development but also have been shown to affect the differentiation of B1, MZ, or Fo B-cell populations. Furthermore, BCR signaling strength may affect antigen-induced B-cell activation, migration, and surface co-stimulator molecule expression levels. It seems that there is still work to be done on how differential BCR signaling influences the differential development of B cells and how the various stages of antigen-induced T–B cell interactions affect further B-cell fate decisions.
This work was supported by grants from the BBSRC UK BB/M025292/1 and a postdoctoral fellowship from the National Council of Science and Technology Mexico (CONACYT-Mexico) to JCY-P (CVU 49896).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Apr 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)