Keywords
retinoblastoma, uveal melanoma, ocular tumors
retinoblastoma, uveal melanoma, ocular tumors
Retinoblastoma and uveal melanoma, albeit rare, are the most commonly observed intraocular malignancies in pediatric and adult populations, respectively. Retinoblastoma occurs during early childhood in 1 per 16,000 people worldwide1, whereas uveal melanoma occurs on average in Caucasians in their fifties and sixties2. Recent advancements in the techniques used to treat these two types of cancer have drastically enhanced patient outcomes and eye salvage rates. In this review, we briefly discuss current treatment guidelines and some emerging topics in the management of primary retinoblastoma and primary uveal melanoma.
Retinoblastoma presents unilaterally in approximately 60–70% of cases and bilaterally in the remaining 30–40%. In unilateral retinoblastoma, close to 90% of patients present with a sporadic mutation, whereas heritable mutations of the RB1 gene (located at chromosome 13q14) with a known affected family member occur in approximately 10% (Table 1)3,4. A small fraction of non-heritable retinoblastoma presents with a MYCN oncogene mutation that results in a unilateral, sporadic tumor5. Unlike unilateral disease, bilateral retinoblastoma is always due to a germline mutation and commonly presents earlier in life than unilateral cases. With the advent of improved sequencing techniques, mosaicism is increasingly being recognized in both unilateral and bilateral patients. The presence of a germline RB1 mutation increases the risk for secondary cancers, especially when retinoblastoma is treated with external beam radiation (EBR)6.
Both the Reese–Ellsworth and the International Classification of Retinoblastoma (ICRB) systems can be used to classify retinoblastoma, although the latter, newer system has been widely adopted in the last decade (Table 2). ICRB divides retinoblastoma into five categories; class A is the least advanced and E is the most advanced type7. Focal therapies such as laser ablation and cryotherapy can be used for retinoblastoma with ICRB classes A and B, whereas more advanced cases (ICRB class C, D, or E) are preferentially treated with systemic chemotherapy or intra-arterial chemotherapy (IAC) over EBR or plaque brachytherapy because of their adverse effects. Enucleation of the eye is performed when there is a potential risk of extraocular extension, especially in class E eyes, or when all prior treatments have failed.
EBR was used as primary therapy for retinoblastoma in most cases until the early 1990s and then intravenous chemotherapy (IVC) until the early 2000s. In 2004, a group of Japanese investigators reported a new technique of balloon-occluding the internal carotid artery distal to the ostium of the ophthalmic artery and then locally injecting melphalan to treat retinoblastoma8. In 2008, Abramson et al. reported a more sophisticated technique of directly infusing melphalan into the ophthalmic artery with a microcatheter that many centers have now adopted with variations9. In this report, seven out of 10 eyes classified as Reese–Ellsworth V and originally scheduled for enucleation were salvaged by IAC. Numerous studies have since reported on the efficacy of IAC compared with that of IVC. In one study by Shields et al.10, global salvage rates for IAC and IVC for class D tumors were 91% and 48%, respectively, demonstrating that IAC can be particularly successful at treating more advanced tumors. Therefore, at many large centers of excellence, IAC is the preferred treatment modality for unilateral and non-hereditary retinoblastoma. Bilateral retinoblastoma with a germline RB1 mutation can be treated with either systemic chemotherapy or tandem IAC, in which IAC is performed in both eyes in a single IAC session11. In case the ophthalmic artery anatomy is not amenable to IAC, the external carotid artery can be alternately used to gain access to the ocular vasculature12. Most large centers have reported superior ocular salvage rates with IAC compared with systemic chemotherapy (Figure 1). Systemic treatment-related immediate effects such as immunosuppression are also rarer with IAC13,14. Clinicians at centers that continue to use systemic chemotherapy have reported concerns about increased risks of metastatic retinoblastoma and risks of secondary cancers15,16. However, these controversies are unresolved with both fierce advocates and staunch opponents of IAC in existence with no clear sign of a definitive multi-center collaborative trial in the works that might settle the debate. As such, there continues to be a heterogeneity of treatment approaches in the US and abroad.
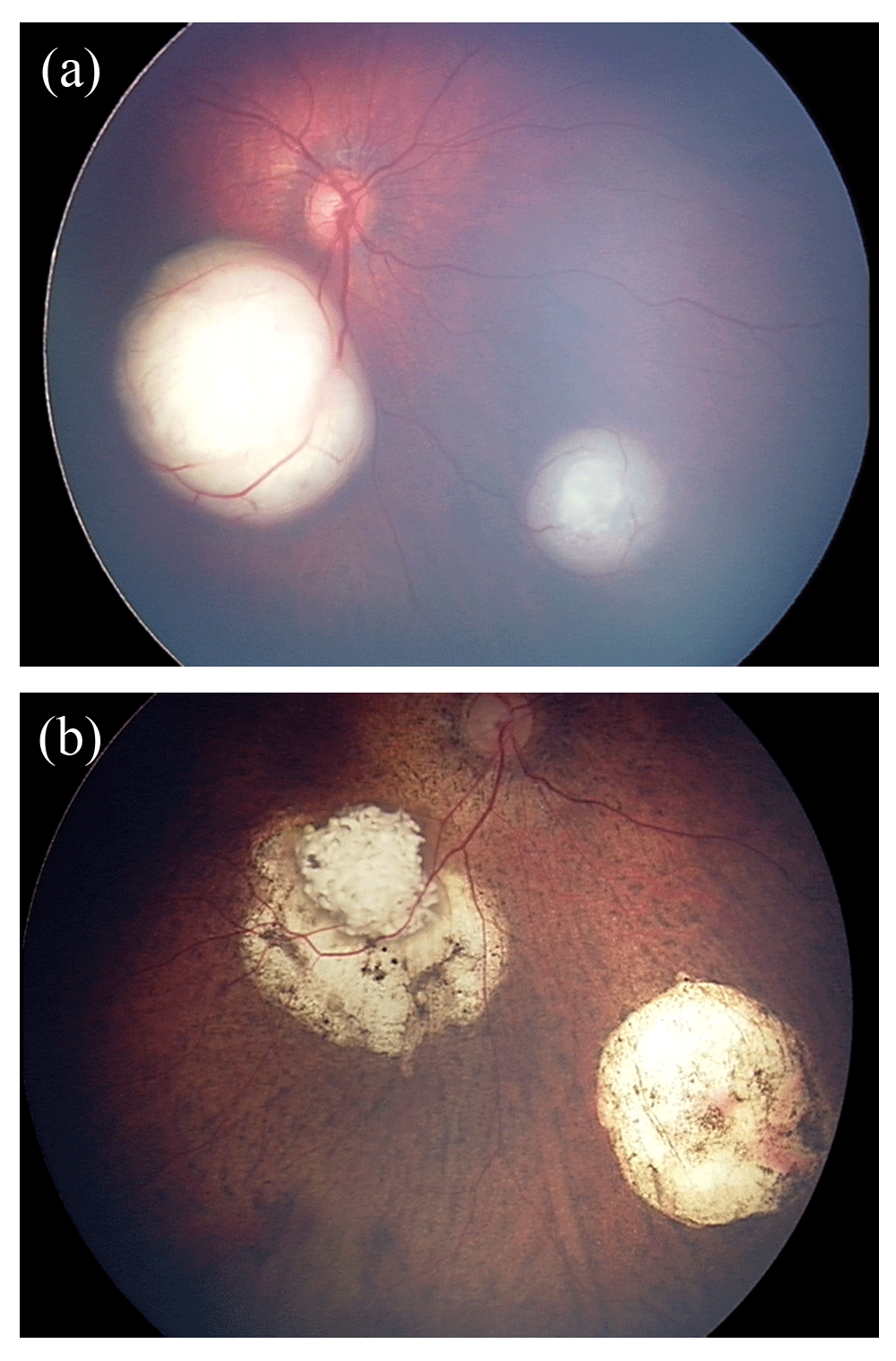
(a) Fundus photograph of the right eye before IAC, demonstrating macular and inferonasal lesions. (b) Fundus photograph of the same eye 13 months after the initial IAC treatment. The patient underwent three IAC cycles and adjuvant therapy, including five sessions of laser ablation and two sessions of cryotherapy.
In many centers, IAC has been widely adopted as the primary therapy for retinoblastoma, and numerous publications have reported successful treatment outcomes. One of the recent reports, by Abramson et al., demonstrated that over 90% of patients now undergo IAC for primary therapy, and the global salvage rate at 48 months post-IAC significantly increased, from 68% in the late 2000s to 92.7% between 2010 and 201417. Shields et al. reported 100% ocular salvage for ICRB class B and C eyes, for which IAC was used as primary treatment18. IAC has also been demonstrated to be highly efficacious for advanced tumors, and 5-year ocular survival exceeded 70% for class D tumors18–20. Class E tumors show mixed results: ocular salvage ranged from 30% to 70% in the literature18–20.
Whereas the safety and efficacy of IAC have been well demonstrated in multiple studies over the past decade, the guideline for age and weight threshold for IAC has not yet been strictly defined. It has generally been assumed that IAC should be reserved until patients with retinoblastoma reach a weight of 10 kg or the equivalent age because of potential procedural complications, such as groin hematomas or femoral artery dissection21. However, several studies have recently demonstrated that patients with retinoblastoma who are younger than 3 months of age can be successfully treated with IAC22,23. The safety of IAC can be further enhanced with ultrasound guidance for femoral artery catheterization, which has long been used in various kinds of pediatric procedures. A pilot study of six patients with retinoblastoma24 demonstrated no procedural complications when IAC was administered with ultrasound guidance to patients with a median weight of 9.2 kg at the first IAC cycle. Recent literature suggests that younger and treatment-naïve patients may achieve better oncologic efficacy when they receive a minimal number of IAC cycles25,26. Also, Gobin et al. reported that eyes that received IAC as primary treatment had an ocular event-free survival rate of 81.7% after 2 years, which was significantly higher than the rate of 58.4% for the eyes that had undergone IVC or EBR prior to IAC27. Therefore, unless there are known contraindications for IAC, such as metastatic retinoblastoma, optimal treatment outcomes may be achieved when the patients undergo IAC at the earliest age possible.
Vitreous seeds are groups of tumor cells that break off from the primary lesion and are commonly seen in advanced retinoblastoma. Vitreous seeds are seen in ICRB class C, D, and E. Dust, spheres, and clouds are the three forms of vitreous seeds with different patterns of response to chemotherapy which have been previously characterized28. IAC has limited efficacy for vitreous seeds because of the avascular nature of the vitreous. Intravitreal injections of chemotherapy via the pars plana have now been widely adopted for persistent or recurrent vitreous seeds after primary treatment with IAC or systemic chemotherapy28–30. In general, intravitreal melphalan or topotecan is combined with a simultaneous treatment of the retinal tumors from which the vitreous seeds originate. To prevent any potential extension of tumor seeds via the needle tract, clinicians typically take various safety measures, including cryotherapy applied to the needle site, visualization of the pars plana with ultrasound biomicroscopy, washing of the ocular surface, and subconjunctival chemotherapy30,31. The safety and efficacy of intravitreal injection of melphalan have been demonstrated in multiple studies28,32.
IVC has been used as primary and secondary therapy for retinoblastoma for over two decades. A three-agent combination (carboplatin, vincristine, and etoposide) is commonly used33, and other agents, including topotecan or cisplatin, can be additionally administered depending on the patient’s response to the agents34. Multiple studies report that over 90% of tumor control was achieved by using IVC, especially for ICRB classes A, B, and C10,35. IVC can be effective at managing both bilateral and germline retinoblastoma. Some investigators have reported that IVC helps in preventing extraocular secondary cancers, including trilateral retinoblastoma in which extraocular cancer occurs in the pineal or suprasellar region36–38; however, this is controversial. There are some rare but recognized side effects of IVC, including neurotoxicity, immunosuppression, secondary leukemia, nephrotoxicity, and ototoxicity34.
Owing to concerns for radiation retinopathy, radioactive plaque brachytherapy is most commonly used as rescue therapy for relatively small solitary tumors in ICRB class A or B39,40. However, plaque brachytherapy is one of the preferred secondary treatment modalities before enucleation. There have been published studies that demonstrated the efficacy of iodine-125 plaque brachytherapy as salvage treatment for retinoblastoma after both IAC and IVC39,40.
Uveal melanoma is a malignant cancer that occurs in 4.9 people per million in the US alone41. As the name suggests, uveal melanoma can occur in any part of the uveal tract, including the iris, ciliary body, or choroid, and the involvement of the choroid is the most common. Uveal melanoma is known to spread hematogenously, and the most common sites of metastasis in descending order are liver, lung, and bone42. Mean overall 5-year survival rate has remained stable at approximately 80% over the past several decades2,43, while the 5-year survival rate drastically decreases once the tumor metastasizes. Lifetime rates of metastases in patients with uveal melanoma are controversial, but rates reported in the scientific literature range from 25% to 50% with a median survival of 6 months to a year after the development of metastatic disease44–46. Some studies have reported a longer median survival once metastatic disease is diagnosed, but other authors claim that lead-time bias explains these results47. Upon initial diagnosis, most patients currently receive plaque brachytherapy, proton beam therapy, or enucleation, except for some iris tumors that can be surgically resected.
Uveal melanoma is largely due to sporadic mutations in uveal melanocytes, and inherited germline mutations that contribute to the development of this tumor are extremely rare, occurring in 3% to 4% of patients48. However, a number of publications since the early 1990s have discussed the importance of cytogenetic changes of the cancer cells which significantly affect the prognosis. Uveal melanoma is histopathologically characterized by spindle and epithelioid cells49. Standard cytology procedures, including cell block analysis with hematoxylin–eosin stain and HMB45/Ki67 immunohistochemical stain, can identify cells acquired from biopsies. Although epithelioid cells are strongly associated with more aggressive behavior, most uveal melanomas contain mixed spindle and epithelioid cells regardless of the predisposed metastatic risk49. One study published that epithelioid and necrotic cell types have a statistically significantly higher rate of 5-year metastatic mortality rate than other cell-type findings50. In the same study, cytopathologic classification was found to be an independent prognostic factor for metastatic death50.
In the early 1990s, Prescher et al. first reported that monosomy 3, the abnormal presence of only one copy of chromosome 3, was a commonly observed cytogenetic abnormality in uveal melanoma51. Since then, a number of studies focusing on the genetics of uveal melanoma have been published. Several key driver genes, including GNA11, GNAQ, BAP1, SF3B1, and EIF1AX, have been identified to be involved in the development and metastasis of the cancer52. Combinations of mutations of these genes lead to variations in the development and metastasis of uveal melanoma. Of these, GNAQ and GNA11 mutations are involved in the early stage of oncogenesis and occur in a mutually exclusive manner in approximately 91% of the patients53. Because these mutations occur early in oncogenesis, neither one confers valuable prognostic information. Recently, a loss-of-function mutation of BAP1, a tumor suppressor gene, was discovered to be heavily associated with more malignant types of uveal melanoma. Loss of BAP1 induces dedifferentiation of melanoma cells and the development of stem cell-like characteristics54,55. On the other hand, hemizygous, gain-of-function mutations of SF3B1 and EIF1AX generally indicate a better prognosis and occur in lower-risk melanomas56. Of note, melanomas with SF3B1 mutations are associated with late-onset metastases57. BAP1, SF3B1, and EIF1AX mutations mostly occur late in tumor development and also occur in a mutually exclusive fashion58.
Gene expression profile (GEP) analysis and multiplex ligand-dependent probe amplification (MLPA) have been adopted by ocular oncologists to elucidate each tumor’s genetic characteristics59,60. GEP testing uses a polymerase chain reaction (PCR)-based 15-gene panel and classifies uveal melanoma as either class 1 (low risk for metastasis) or class 2 (high risk for metastasis)59,61. Class 1 is further divided into 1A and 1B; 1A tumors remain relatively low-risk for metastasis, whereas the risk of metastasizing in 1B appears to be higher than the 1A group over time. The 5-year published metastatic rates for class 1A, 1B, and 2 tumors are 2%, 21%, and 72%, respectively58. It has been observed that class 1B uveal melanoma, though categorized under class 1, behaves more similarly to class 2 tumors and therefore requires close monitoring for progression to metastasis. BAP1 somatic mutations are observed predominantly in class 2 tumors, whereas SF3B1 or EIF1AX mutations are seen more frequently in class 1 tumors54. It is reported that BAP1 mutations can be observed in approximately 80% of metastatic uveal melanoma cells54. In another study, 71%, 11%, and 0% of patients with primary uveal melanoma who developed metastases carried BAP1, SF3B1, and EIF1AX mutations, respectively, signifying that EIF1AX and SF3B1 mutations generally confer a good prognosis62. In the largest single-institution case series of over 1,000 patients, 3-year Kaplan–Meier estimates for metastatic uveal melanoma were provided for the following cytogenetic abnormalities: 5% for partial monosomy of chromosome 3; 19% for complete monosomy 3; 23% for loss of 6q; 29% for loss of 8p; 21% for gain of 8q; 1% for disomy of 3, 6, and 8; 29% for complete monosomy 3, 6p gain, and 8q gain; 14% for complete monosomy of 3, disomy of 6, and gain of 8q and 8p; 27% for complete monosomy of 3, disomy of 6, and gain of 8q; and 28% for complete monosomy of 3, disomy of 6, gain of 8q, and loss of 8p63.
Recently, scientists have discovered that certain uveal melanomas that express a cancer-testis antigen called preferentially expressed antigen in melanoma (PRAME) are closely associated with an increased risk of metastasis in both class 1 and 2 uveal melanomas64,65. Also, class 1 tumors that are PRAME+ were found to be associated with SF3B1 mutations and inversely to EIF1AX mutations65. A combination of SF3B1 mutations and PRAME expression appears to contribute to late metastases in class 1 tumors66, while PRAME+ class 2 tumors exhibited accelerated progression to metastases65. PRAME is currently being investigated as a potential target for immunotherapy in primary and metastatic uveal melanoma67. The Collaborative Ocular Oncology Group 2 (COOG2) is a currently enrolling multi-center prospective clinical trial in which PRAME genomics will be examined along with long-term clinical outcomes.
As research in melanoma genomics has grown explosively, safe and adequate acquisition of tumor cells has become increasingly important for both clinical and research purposes. Fine needle aspiration biopsy (FNAB) is performed by using small-sized needles (23-, 25-, or 27-gauge) or vitrectomy probes in either a transvitreal or a trans-scleral manner, depending on the tumor location. For tumors that are anterior to the equator with direct access to the needle, the trans-scleral method is typically chosen. For posterior tumors that are more difficult to access via a trans-scleral biopsy, transvitreal biopsy can be performed by using indirect ophthalmoscopy or standard retinal instrumentation, including chandelier lighting that gives direct visualization, and valved trocars, which serve to maintain the intraocular pressure during biopsy and prevent the tracking of tumor cells along the needle tract. After the aspiration of tumor cells, cryotherapy is applied to the needle insertion site in order to prevent any iatrogenic extraocular extension of tumor cells via the needle tract. Safety of FNAB for uveal melanoma was recently reaffirmed in a prospective, in vivo study68. Many studies have demonstrated high cellular yield rates, ranging from 68% to over 90%, for cytopathologic and genomic analyses69–73.
The Collaborative Ocular Melanoma Study (COMS) found no statistically significant difference in survival between patients who underwent plaque brachytherapy and patients who underwent enucleation74. Since then, most centers have adopted plaque brachytherapy as the standard treatment for uveal melanoma. Multiple types of isotopes are used for ophthalmic brachytherapy. In the US, 125I is the most frequently used radioisotope after the COMS study, whereas 106Ru and 103Pd are more commonly used in Europe and other countries75. 125I and 103Pd both emit low-energy gamma rays and thus cause less damage to surrounding healthy tissues compared with isotopes that were used in the first half of the 20th century, such as 60Co. 106Ru, on the other hand, emits beta rays and has a quicker dose fall-off75. The steeper the dose gradient, the more concentrated the radiation effect on the basal side of the tumor and conversely less radiation toward the apex. 106Ru has an advantage of less radiation effect to other ocular structures compared with that of 125I or 103Pd.
Multiple studies have demonstrated that plaque therapy and enucleation result in comparable mortality rates over 20 years of follow-up76. Iodine-125 brachytherapy has become the most commonly used treatment modality for uveal melanoma in the US with excellent clinical outcomes (Figure 2).

(a) B-scan ultrasound image of the right eye before the plaque implantation. (b) B-scan ultrasound image of the same eye intraoperatively, demonstrating full coverage of the tumor with the plaque. (c) B-scan ultrasound image of the same eye 3 years after the plaque therapy, demonstrating regression of the tumor.
Local recurrence of tumor cells at the ocular site is a critical complication to be avoided after plaque therapy. Multiple studies have demonstrated that the likelihood of metastasis increases dramatically after local recurrence occurs77,78. However, the 5-year local recurrence rate has steadily decreased from 10.3% at the time of the COMS76 to 2.4% to approximately 4.7%77,79 over the past two decades. Indeed, a recent publication that reported preliminary clinical outcomes with a median follow-up of 21.6 months80 demonstrated zero local recurrence, which may be attributed to several factors. First, newer plaque designs81,82 are thinner than the traditional COMS plaques and are customized to conform better to each patient’s eye, leading to better coverage of the tumor and less radiation scatter outside the targeted area. Second, intraoperative ultrasonographic confirmation of plaque positioning, which has been used more over the past decade, ensures precise placement of the plaque83–85. A recent study at the Cleveland Clinic reported that plaque treatment failure decreased from 9.3% to 1.5% since intraoperative ultrasound was adopted86. Intraoperative transillumination of the tumor and preoperative 3D planning with Plaque Simulation software can further enhance the accuracy of plaque placement80,87. Treatment outcomes of plaque brachytherapy, including 5-year mortality and local recurrence rates (4%), are comparable to those of proton beam radiotherapy in recent publications88,89. Both plaque and proton beam therapy are known to cause ocular complications, including cataracts, radiation retinopathy, and radiation optic neuropathy. The COMS demonstrated that nearly 50% of patients who receive plaque brachytherapy had significant vision loss by 3 years post-treatment because of these complications90.
In addition to the fact that GEP class 2 melanomas have higher mortality rates than GEP class 1 tumors, several additional factors that contribute valuable prognostic information have recently been identified. Correa and Augsburger recently reported that the largest basal diameter (LBD) of the tumor can serve as an independent prognostic factor for metastasis and metastatic death91. Harbour et al. reported that class 2 tumors with an LBD over 12 mm had a significantly lower 5-year metastasis-free survival92. Also, increased patient age, larger tumor apical height, and ciliary body involvement of the tumor are associated with metastatic risk93,94. Traditionally, tumors with more malignant characteristics, such as tumors with monosomy 3 or those that metastasized, were reported to regress faster after plaque therapy95,96. In recent studies, the relationship between GEP class and the tumor regression rate after brachytherapy has been controversial. Whereas several studies97,98 found neither GEP class to be significantly associated with regression rate, another study reported that class 1 tumors regress faster99. In the largest multi-center, retrospective cohort study that was recently published, investigators also reported that class 1 tumors regress at a statistically significantly faster rate than class 2 tumors after plaque radiation100. Future multi-center studies will help elucidate a clearer relationship between GEP class and therapeutic response to radiation.
Some recent studies have identified several key molecular pathways associated with specific genetic mutations. For example, the BAP1 gene is known to regulate histone H2A function by removing ubiquitin molecules. When the BAP1 gene is mutated, proper removal of ubiquitin from H2A is inhibited, leading to a dedifferentiated state of melanoma cells101. Also, GNAQ and GNA11 genes are closely related to transmembrane cell signaling52. Activation mutation of GNAQ or GNA11 keeps guanine nucleotide-binding proteins in an active state, which subsequently upregulates protein kinase C and mitogen-activated protein kinase pathways that are involved in the proliferation and differentiation of cells at the early stages of uveal melanoma oncogenesis102. Many ongoing clinical trials (some of which are still accruing patients and some of which are now closed to new patient enrollment) are examining immunotherapy agents that target these pathways as well as several others for both high-risk and metastatic uveal melanoma (Table 3 and Table 4).
Nanoparticle therapy is an emerging cancer therapy, in which photosensitive nanoparticles preferentially bind tumor cells, followed by light activation of the nanoparticles103. This is a minimally invasive yet highly specific treatment modality that can kill tumor cells with minimal damage to the surrounding normal tissues. For uveal melanoma, a phase 1b clinical trial has begun to investigate the safety of a new nanoparticle phototherapy for small to medium-sized tumors in 12 patients (http://www.aurabiosciences.com/news-archive/2017/3/30/aura-biosciences-announces-initiation-of-phase-1b-clinical-trial-and-receipt-of-fda-fast-track-designation-for-au-011-for-the-treatment-of-primary-ocular-melanoma). Viral nanoparticle conjugates attach to the uveal melanoma cell membrane. When activated by a 589 nm laser, the particles selectively break down the tumor cell membrane without affecting adjacent tissues. This treatment modality, if proven successful in clinical trials, has the potential to preserve much of the patient’s vision and could be particularly groundbreaking in patients with small tumors that are close to critical ocular structures such as the optic nerve and the macula. The effect on rates of metastatic disease are still unknown.
Extensive advancements have been made in the understanding and treatment of retinoblastoma and uveal melanoma over the past decade. Further knowledge of intraocular cancer genetics will lead to new clinical breakthroughs that will allow us to save more eyes and lives.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: Aura Biosciences – Clinical Advisor ICONIC Therapeutics – Consulting Castle Biosciences – Consulting
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Apr 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)