Keywords
Chlorella sp., cadmium removal, immobilization, alginate
Chlorella sp., cadmium removal, immobilization, alginate
Pollution from the use of metals as a consequence of waste generated by industries is a constant concern, since they may end up being transferred to the environment1,2. The need to develop technologies for the remediation of water contaminated with cadmium should be considered as a priority in Ecuador. The objective of this study was to evaluate the capacity for cadmium removal in aqueous solutions by immobilized Chlorella sp. in calcium alginate beads. This study used the micro-algae Chlorella sp., for the bio-removal of cadmium, since the use of cells of Chlorella sp. immobilized in beads of alginate has been successfully exploited3 and its potential use has been established in the bioremediation of contaminants such as metals4, nutrients5 and other industrial pollutants6.
The assay was carried out with aqueous solutions of cadmium. This solution was placed together with the alginate beads in Florence flasks. Every 10 minutes a sample of the aqueous solution was taken (10 mL approximately) with a glass pipette, and each sample was placed in a test tube. The concentration of metal was measured by aspiration atomic absorption spectrophotometer.
To make the control beads, the procedure described in a previous work was followed7. Briefly, 2 grams of sodium alginate (Loba Chemie, high density) were dissolved in 200 mL of ultra-pure water to obtain a homogeneous mixture, adjusting the pH between 7.8–8.0. pH was adjusted by addition of 0.1 molar sodium hydroxide or 0.1 molar hydrochloric acid, in sodium alginate paste (homogeneous mixture). One drop at a time was slowly poured into a 1% w/v calcium chloride solution with constant stirring.
For the immobilization of Chlorella sp. in alginate beads, liquid cultures were used. The volume of culture to be used was established to reach a concentration of 25 X 106 cells mL per each mL of alginate. For the preparation of the beads, 2 g of sodium alginate were dissolved in microalgae culture, as above.
Experimental liquids were placed in 12 Florence Flasks of 500 mL capacity, inside which were placed 50 g of alginate beads without immobilized Chlorella sp. and 200 mL of cadmium solution at different concentrations (0, 20, 100 and 200 ppm). Each concentration was performed in triplicate, essay was evaluated during 80 minutes, aqueous solution samples were taken at 10 min intervals. Each Florence flask was placed on a shelf in the laboratory, the location of each bead on this surface was performed randomly. The flasks were subjected to constant aeration with air pressure of 0.012 MPa for 80 min. Every 10 minutes, an aqueous solution sample was taken to measure the concentration of metal present. These were the control treatments.
For the experimental samples, 50 g of alginate beads with immobilized Chlorella sp. were placed in each flask with the same concentrations of cadmium solution as the controls. These experiments were also performed in triplicate. These experimental samples were used to establish whether the addition of Chlorella sp. in the beads, increases or decreases the alginate removal activity.
The determination of metal concentration in the solutions was measured with a VARIAN direct aspiration atomic absorption spectrophotometer (VARIAN. Model: SpectrAA 55, Wavelength: 326.1 nm), using air-acetylene flame with cadmium lamp, and results are presented as % of removal of cadmium.
For viability tests, color change of the cultures exposed to cadmium was evaluated. For this purpose 100 mL of liquid Chlorella cultures were used (25 × 106 cells/mL) and they were subjected to different concentrations of Cd (0, 20, 100 and 200 ppm), in triplicate. The color change was monitored at the first hour of treatment, at 24 hours and at 7 days.
Color changes were observed in the solutions (Figure 1, Dataset 1 and Dataset 2) of free micro-algae cells exposed to different concentrations of cadmium, which is a product of the toxicity of the metal, while the solutions without the presence of metal maintained a green color, characteristic of Chlorella sp.7

subjected to different concentrations of Cd, at 60 minutes, (A) 0 ppm Cd, (B) 20 ppm Cd, (C) 100 ppm Cd, (D) 200 ppm Cd. Green color means that algae remain viable, brown color means that Chlorella loses viability.
It was determined that Chlorella sp. exposed to a concentration of 20 ppm cadmium at 60 minutes of contact does not react, and shows no noticeable changes in coloration. On the other hand, the micro-algae did not show resistance to concentrations 100 and 200 ppm of Cd, revealing that the cells did not remain viable due to the color variation and high toxicity of the metal used, going from green to a grayish brown in the first 60 minutes of the start of the trial. These results show to decrease algal growth and inhibit photosynthesis8 100 ppm or higher.
It is verified that the best treatment for Cd removal is the one corresponding to alginate beads with immobilized Chlorella sp. at the concentration of 20 ppm of Cd at 80 min. It was determined that at low metal concentrations the micro-algae enhances its removal capacity achieving a removal percentage of 59.67% with Chlorella sp., being significantly higher (p value <0.001) than the removal presented without Chlorella sp. (55.56%), explained by the viability process that showed that Chlorella sp. can withstand the toxicity of the metal for periods of at least 60 min at concentrations of 20 ppm (Figure 2 and Figure 3). As demonstrated by 9, micro-algae work better at lower concentrations of metal.
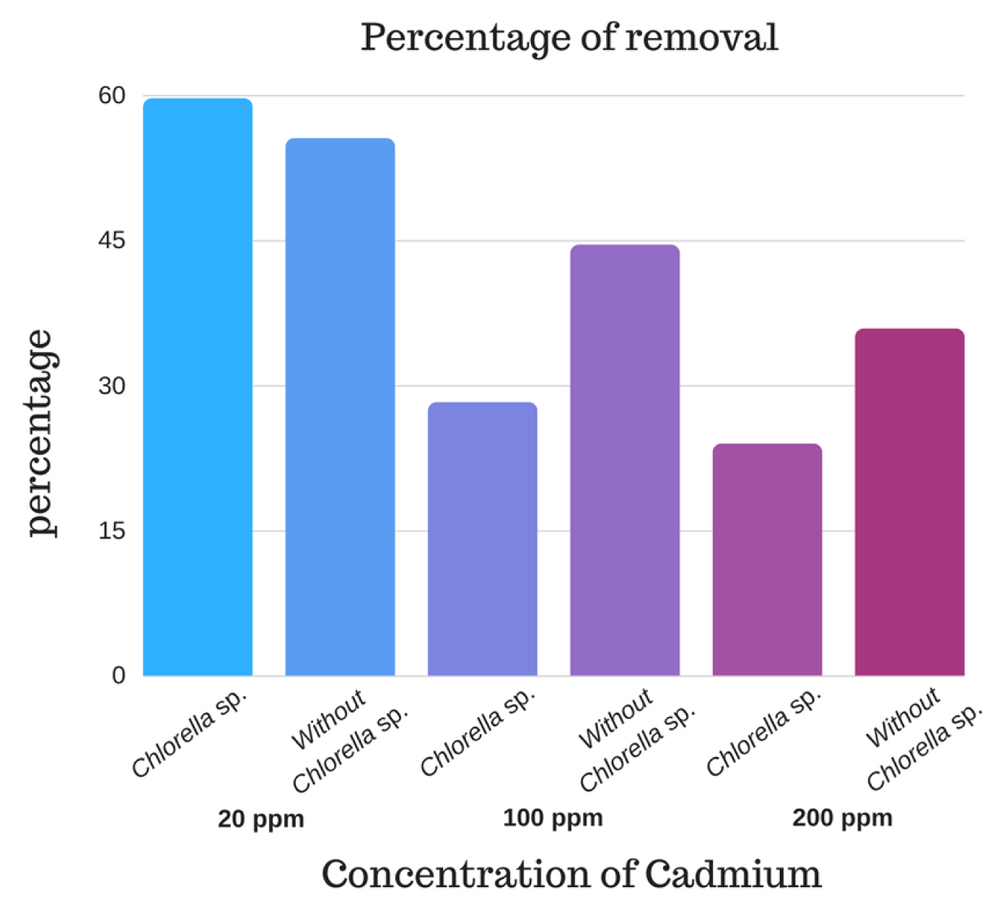
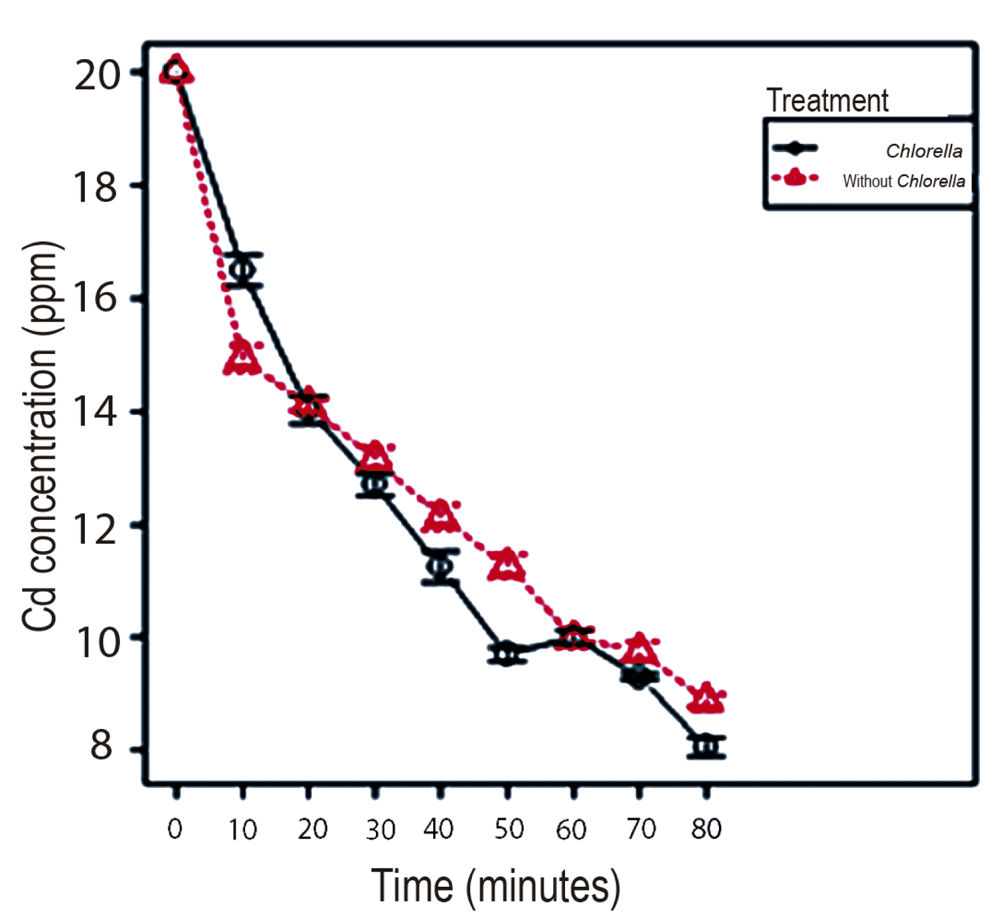
Chlorella sp. immobilized in alginate beads could be used for bioremediation processes of cadmium at low concentrations of the metal, since the presence of viable biomass of the micro-algae potentiates the removal capacity of the alginate. Non-viable Chlorella sp., because of the high concentration of cadmium (100 and 200 ppm), decreases its removal capacity, yielding better results in beads treated without Chlorella sp. Thus, the alginate matrix without micro-algae could be used effectively in Cd removal processes at high metal concentrations.
Dataset 1. Percentage of Cd in aqueous solution at 80 minutes. DOI, 10.5256/f1000research.13527.d19026610
Dataset 2. Raw data for the percentage removal of cadmium with and without Chlorella alginate beads at all concentrations and triplicate experiments DOI, 10.5256/f1000research.13527.d19028111
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental technologies
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Applied chemical ecology and applied organic chemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Jan 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)