Keywords
Ambrosia Arborescens, Aristeguietia glutinosa, essential oil, extract, antifungal, dermatomycosis
This article is included in the Pathogens gateway.
Ambrosia Arborescens, Aristeguietia glutinosa, essential oil, extract, antifungal, dermatomycosis
This research provides a technical basis that allows for plants used in the field of ethnomedicine to be used in the development of natural products in concentrations that allow for an effective therapeutic action. Plants have been identified as being useful in treating several diseases that have affected populations since ancient times, amongst which are allergies, pains, infections and skin diseases. The most studied skin lesions are related to different forms of dermatitis, skin allergies, acne and bacterial infections of the skin. Several medicinal plants, or essential oils and natural extracts obtained from them, have a curative influence on these pathologies1.
Many plants have been granted the classification of "medicinal", being part of the intangible natural heritage of a country, thus it is advisable to take advantage of ancestral knowledge2 in the development of new therapeutic agents. Current knowledge about medicinal plants has two fundamental objectives, once their therapeutic value has been validated. The first is to discover the molecular structure of the active principles and the second is to lay down scientific bases for the direct use of the plants by large sectors of the population3. Ambrosia arborescens (Marco) and Aristeguietia glutinosa (Matico) belong to the Asteraceae family, which are characterised as being aromatic plants with medicinal properties. Marco is widely used amongst the inhabitants of our region in order to mitigate headaches, migraines, rheumatism, fever, constipation, prostate disorders, fractures and injuries as well as for vaginal baths4. Within agriculture, it is used as a biological means of controlling pests, related to the protection or defense against attacks of microorganisms and insects5. Matico is a native species of the Inter-Andean valley and is found only in Ecuador. It is attributed with having anti-inflammatory, expectorant, antitussive, healing and disinfectant properties. It is also used as an emollient and protector of the skin6.
In the same way that the nutritional value of certain plant products has been discovered over the years, humankind has also determined the curative or analgesic value of many plants. The extinction of plants and loss of cultural knowledge puts at risk new applications which have not yet been developed by modern science and might be valid, safe and effective5.
Synthetic drugs offer indisputable advantages, however it has been found that undesirable and even serious sideeffects can occur. This has encouraged a return to the study of plants in an attempt to find natural drugs that have higher therapeutic value and lower pathological risks. Although empirical knowledge is valuable, it is not reliable enough, thus scientific confirmation is necessary3. In the case of dermatoses, the most commonly used medication for lesions is Clotrimazole (a compound derived from the imidazole family) commonly used for the treatment of yeast infections, oral candidiasis, and dermatophytosis (ringworm). It is also used to treat athlete’s foot and tinea corporis7.
Another problem is the innate and acquired resistance that has been demonstrated in vitro and in vivo, therefore the early recognition of species resistant to one or more antifungals guarantees an optimal treatment and better result. However, resistance is constantly growing and evolving, so the introduction of new antifungals is important8.
The fungi that produce superficial mycoses are opportunistic, thus patients with one or more of the following are more susceptible: diabetes, AIDS, cancer and any other debilitating or chronic condition. Worldwide, a prevalence of 5% to 10% of mycosis caused by Candida has been found by dermatology services. Other isolated species with greater frequency are Tinea corporis and Tinea capitis as well as the following fungi: Microsporum canis (9%), Trichophyton mentagrophytes (8.8%) and Trichophyton rubrum (8.6%)9.
The ethnobotanical use of species such as Marco (Ambrosia arborescens) and Matico (Aristeguietia glutinosa) in skin conditions allows them to be considered inhibitory agents of pathogenic fungi. The verification of this activity in vitro allowed us to determine the most effective concentrations and consequently one could standardise the use of these species as natural assets in the formulation of products for the treatment of dermatomycosis.
The vegetal material was collected in Ibarra City in the province of Imbabura. Ambrosia arborescens was obtained from the surrounding area of the Yaguarcocha Lagoon and Aristeguietia glutinosa in the San Miguel de Arcángel neighbourhood on the Alto de Reyes Hill. Ibarra is 2225 metres above sea level (m.a.s.l.). Located 115km northwest of Quito and 125km south of the city of Tulcán, it has a temperate and pleasant dry climate. The historical meteorological annuals have determined an average temperature of 15, 90°C, with a minimum variation of less than 0, 3°C.. Records show a maximum mean temperature of between 20 and 25°CC and a minimum mean of between 7 and 11°CC (see Table 1). Maximum average wind speeds are 7m/s and the minimum are 3.5 m/s. A hydrometeorological analysis has determined that average annual precipitations are between 1000 mm and 1400 mm10.
| Ambrosia arborescens | Aristeguietia glutinosa | |
|---|---|---|
| Location | Laguna de Yaguarcocha | Loma Alto de Reyes |
| Coordinates | 022°’0" N 78°7’0" W | 0° 21’46"N 78°07’48"O |
| m.a.s.l | 2190 | 2405 |
| Temperature | 15°C | 17°C |
| Humidity | 72% HR | 68% HR |
Data was obtained on the day of collection is presented in Table 1.
The material was dried under referenced conditions that guarantee not to affect it, namely that air temperature should be moderated to avoid the temperature of the herbs exceeding critical temperature (generally between 30 and 60°C for medicinal plants). Drying interrupts the degradation processes caused by enzymes or ferments and prevents the development of microorganisms and oxidation and hydrolysis reactions11.
Of all the fungal population, with regard to the group of fungi and complying with international regulations, depending on the incidence of skin diseases9, the in vitro analysis was carried out on the following microorganisms: Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis and Candida albicans, which are fungi with high influences on treated dermatomycoses.
We worked with two types of extracts. We used a soft extract for Marco (Ambrosia arborescens), obtained by placing percolation in 96% ethyl alcohol (1:4) for 72 hours at room temperature, with a subsequent evaporation in a vacuum until a consistency of thick stringy mass was achieved (15 to 25% humidity) and the concentration is 2:1 according to Osorio (2009)12. In the case of Matico (Aristeguietia glutinosa), a steam distillation at 92°C was carried out in a distiller for 45 minutes until the essential oil was obtained.
The method is based on the relationship between the concentration of the substance needed to inhibit a bacterial strain and the growth inhibition halo on the surface of an agar plate with a suitable culture medium, planted homogeneously with the bacteria to be tested and upon which a 6mm diameter filter paper disc has been placed, or planted in an impregnated well with the correct amount of the substance13. The Microbiologics-KWIK-STIKTM strains Trichophyton mentagrophytes ATCC 9533, Trichophyton rubrum ATCC 28188, Microsporum canis ATCC 36299 and Candida albicans ATCC 10231 were used for the evaluation. We carried out an activation of the lyophilized strains and a subsequent culture in the first three microorganisms. We prepared a suspension of spores, evaluating the concentration of them with the Neubauer count chamber and the planting value or inoculum for the three microorganisms was 106. In the case of Candida albicans ATCC 10231, the preparation of the inoculum was carried out by means of the method for the quantification of the growth of microbial populations based on the cell mass by tubidimetry using a UV Shimadzu Spectrophotometer (model: mini 1240). The bibliographically referenced conditions determine a wavelength of 625 nm, an absorbance value of 0.08 to 0.11 which is equivalent to 1-2x108 UFC ml for bacteria and a wavelength of 530nm and absorbance value of 0.13 to 0.16 which is equivalent to 1-5x106 cells for fungi and yeasts14. Seven successive dilutions of Ambrosia arborescens (Marco) extract. Concentrations of 100ppm, 200ppm, 300ppm, 400ppm, 500ppm, 600ppm and 700ppm (parts per million) were made using dimethyl sulphoxide (DMSO) polar miscible fluid and without antifungal activity as diluent. 10 dilutions of Aristeguietia glutinosa (Matico) essential oil were made in percentages of 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5%, 5% and 100% using Dimethylsulfoxide (DMSO) as the diluent. Planting was executed in plates by mass homogenisation for Trichophyton mentagrophytes, Trichophyton rubrum and Microsporum canis. 1ml of inoculum of the fungus was deposited and 19 ml of molten Sabouraud Dextrose Agar (SDA) culture medium was added, tempered at approximately 45°C and sterile. Both were mixed by gentle rotation of the plates. In this way, the microorganisms were distributed homogeneously in the culture medium allowing growth throughout the agar15.
Once the inocula were planted, the natural extracts were placed in the indicated concentrations following the Kirby-Bauer methodology, a method which was modified for use with diffusion in agar wells. The wells were made on the surface of the agars with the help of a sterile hole puncher with a diameter of 6mm. Then, 50µl of different concentrations of extract, different concentrations of oil, as well as a positive blank (clotrimazole) and negative blank (Dimethylsulfoxide-DMSO) were poured into each of them. The plates were incubated for 2 to 5 days at 25°C to allow confluent fungal growth. All tests were done in triplicate. The inhibition halos were measured with a gauge or calliper (mm), considering them as starting from the point of complete inhibition as seen by the naked eye. The reading was taken on the back of the plate with a black background and reflected light. The interpretation of the reading was based on the Kirby-Bauer method, which relates the concentration of the substance necessary to inhibit a bacterial strain with the growth inhibition halo on the surface of an agar plate with a suitable culture medium and seeded homogeneously with the tested fungi and on which has been deposited a (6 mm diameter) filter paper disk16.
The statistical analysis was carried out with a two-way ANOVA and the Tukey Test (HSD) with the use of the Statistix 10.0 General AOV/AOCV Program and the Statistix 10.0 Tukey HSD All-Pairwise Comparisons Test Program.
Table 2 shows the results of an average of three determinations whose deviation limit is close to 95%. The results showed antifungal activity in each of the concentrations and the positive blank (Clz) used on Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis and Candida albicans.
Table 3 shows the results of an average of three determinations whose limit of deviation is close to 95%. The results evidenced antifungal activity in each of the concentrations and the positive blank (Clz) used on Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis and Candida albicans.
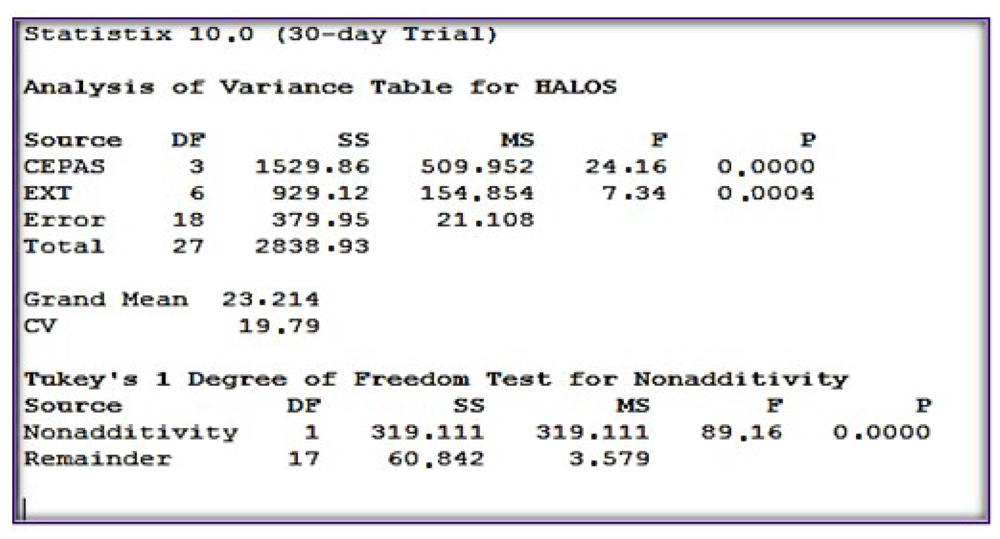
The two-factor ANOVA statistical analysis resulted in a probability of p=0.0004 which is less than α (0.05). The statistical test F is 8.23, higher than the Ft of 5.41. These values allow one to accept the alternative hypothesis, which is that Matico (Aristeguietia glutinosa Lam.) EO presents antifungal activity (Figure 1). Once the alternative hypothesis was accepted, the Tukey test was carried out a posteriori to demonstrate whether or not there is a significant difference between the inhibition halos.
The test evidenced the existence of two groups: A and B. Group A consisted of Microsporum canis and group B consisted of Candida albicans, Trichophyton rubrum and Trichopyton mentagrophytes. The most evident inhibition average was seen on Microsporum canis with an average of 20.81mm (Table 4).
The two-factor ANOVA statistical analysis resulted in a probability of p=0.000 which is less than α(0.05) and the statistic of the F test is 24.16, higher than the Ft of 4.76. These values allow one to accept the alternative hypothesis, which is that extract of Marco (Ambrosia arborescens Mill.), presents antifungal activity (Figure 2). Once the alternative hypothesis was accepted, the Tukey test was carried out a posteriori to show whether or not there is a significant difference between the inhibition halos.
The test evidenced the existence of three groups: A, B and C. Group A consisted of Microsporum canis and Trichophyton rubrum, group B of Trichophyton rubrum and Trichopyton mentagrophytes and group C of Candida albicans. The most evident inhibition average was seen on Microsporum canis with an average of 31.20mm (Table 5).
The evaluation of the antifungal activity of the Ambrosia arborescens extract showed inhibition in the studied dermatophytes in each one of the planted concentrations (100 to 700ppm). The data discrimination of the Tukey test, after the one-way Anova, showed that the most effective concentrations were at 700ppm for Trichophyton mentagrophytes and Trichophyton rubrum and 600ppm for Microsporum canis and Candida albicans. The two-way Anova statistical analysis proved that the extract presented a greater homogeneity at concentrations of 300 and 400ppm over the studied dermatophytes in comparison to the other concentrations, obtaining an average halo of 23.37mm. The evaluation of the antifungal activity of Aristeguietia glutinosa EO showed inhibition in the studied dermatophytes in each of the planted concentrations (0.5 to 5%). The data discrimination from the Tukey test, after the one-way Anova, showed that the most effective concentrations were 5% for Trichophyton mentagrophytes, 4.5% for Trichophyton rubrum, 5% for Microsporum canis and 2% for Candida albicans. The two-way ANOVA (analysis of variance) proved that the EO has a greater uniformity of inhibition at 2, 2.5 and 3% in relation to the other concentrations, for which an average halo of 15.75mm was determined on the studied dermatophytes.
Dataset 1. Diameter of inhibition halos of Ambrosia arborescens extract. Diameter (mm) of inhibition halos of Ambrosia arborescens EO. positive control (Clz +) and negative control (DMSO -) used on Trichophyton mentagrophytes. Trichophytonrubrum. Microsporum canis. Candida albicans. 10.5256/f1000research.14354.d20149217
Dataset 2. Diameter of inhibition halos of Aristeguietia glutinosa essential oil against fungus. Diameter (mm) of inhibition halos of Aristeguietia glutinosa essential oil. positive control (Clz +) and negative control (DMSO -) used on Trichophyton mentagrophytes. Trichophyton rubrum. Microsporum canis. Candida albicans. 10.5256/f1000research.14354.d20149318
Matteo Radice confirms that the author has an appropriate level of expertise to conduct this research, and confirms that the submission is of an acceptable scientific standard. Matteo Radice declares they have no competing interests. Affiliation: Faculty of Agroindustry and Faculty of Agricoltural and Zootechnics, Universidad Estatal Amazonica, Puyo, Ecuador.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Mycology, parasitology
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 May 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)