Keywords
Gastroparesis; macrophages; gastric emptying
Gastroparesis; macrophages; gastric emptying
Eating not only ensures survival but also is part of the social fabric. Meals often function as times for social interaction, as symbols of hospitality, or as a central aspect of celebrations. Thus, illnesses that limit the ability to partake in this important daily activity have a disproportionately high impact on quality of life1. About 60 years ago, Kassander coined the term gastroparesis, describing it as a consequence of vagal neuropathy in diabetics with secondary complications2. We have since gained significant insight into the normal and abnormal function of the stomach, developed and standardized a disease definition and diagnostic approaches, and employed a wide range of treatments from diet to medications and endoscopic and surgical therapies. Current consensus statements highlight the importance of delayed gastric emptying, typically documented by measuring the exit of a solid test meal from the stomach, and the chronic presence of dyspeptic symptoms, such as nausea, vomiting, post-prandial fullness, early satiety, anorexia, and abdominal discomfort3. Yet, given the repeatedly disappointing results of interventions that target disease-defining mechanisms, such as motilin or ghrelin agonists4–8, are we still facing a “conundrum”, as Longstreth lamented more than a decade ago9? Since then, several investigations have shifted our understanding of the start of gastroparesis and this has affected clinical practice.
As mentioned above, gastroparesis is defined by a delay in gastric emptying, which differentiates it from functional dyspepsia or chronic unexplained nausea. The impaired emptying has traditionally been the target of treatments and often functioned as a key endpoint in clinical trials5,6. Yet gastric emptying does not consistently correlate with symptom severity, whether assessed with a labeled meal or a large undigestible particle, such as the wireless motility capsule10,11. This limited correlation could be due in part to a focus on the wrong endpoint, such as half-emptying time, rather than the fraction of a test meal retained after a predefined time12. The relationship between gastric emptying and symptoms improved when acute, meal-induced rather than recall-based severity ratings covering a longer period were used13–15. These chronic complaints and acute, experimentally evoked symptoms are conceptually related; however, trial endpoints and drug approval criteria target the chronic symptoms as clinically validated and relevant endpoints. Interestingly, a recent cohort study described similar symptoms and symptom severity between type 1 and type 2 diabetics, even though gastric emptying was slower in type 1 diabetes, thus similarly pointing away from this variable as the sole or even primary determinant of disease severity16. Conversely, the perceived benefit of treatments does not correlate with changes in gastric emptying17. Lastly, gastric emptying does not reliably do what it is supposed to do as a biomarker, namely differentiate patients with overlapping symptoms but different disorders. Close to 90% of patients with gastroparesis meet the consensus definition of functional dyspepsia and about 20% of patients with functional dyspepsia have impaired emptying independent of their symptom pattern10,18. Similarly, a recent study characterized patients with chronic unexplained nausea, which phenotypically overlapped with gastroparesis except for the normal results of gastric-emptying studies19. The overlap goes beyond symptoms and changes in emptying as detailed physiological studies showed coexisting changes in accommodation and sensory mechanisms in gastroparesis20. If we look at mechanistic studies, we still see more parallels than differences as patients with otherwise-unexplained nausea and vomiting share some of the microscopic abnormalities on full-thickness gastric biopsies that otherwise have been associated with gastroparesis21. Looking for alternative explanations related to gastric motor function, a small study suggested a potential correlation between symptoms and integrated motor activity22, a finding that subsequent investigations did not confirm in a substantially larger cohort with 209 participants11. Thus, we may deal with a spectrum of abnormalities that overlap in symptoms, test results, and underlying pathophysiology. Instead of making gastric emptying a key criterion for diagnosis and treatment, we may reconceptualize dyspeptic syndromes as a potential consequence of several coexisting mechanisms that contribute to a problem. Delayed emptying can clearly be one of these changes and may contribute to symptoms or symptom severity, especially the severity of nausea and vomiting18 or post-prandial symptom exacerbations13–15. Some recent data also point to the potential prognostic value of gastric-emptying data with apparently counterintuitive results, as slower emptying at baseline was associated with a better prognosis after close to one year of follow-up23.
Animal models and human data have identified a network of macrophages in the muscular layer of the stomach, which may link the immune system to changes in gastric motor function. Activation of M1 macrophages triggers the release of tumor necrosis factor alpha, which, in turn, leads to a decrease in interstitial cells of Cajal (ICCs), the electrical pacemaker cells of the gut24. Conversely, interleukin-10 activates M2 macrophages, which protect the ICC population25,26. With animal models showing up- or downregulation of ICCs through such mechanisms and associated changes in gastric emptying7, do we need to look at this motility disorder as a problem at the interface between the immune system and neuromuscular transmission? Bharucha and colleagues recently explored this question by trying to induce heme oxygenase-1, which is expressed in M2 macrophages and plays an important anti-inflammatory role8. Their pilot data show a potent induction of the enzyme but no influence on symptoms or emptying28. Even if macrophages or specific enzymes have not yet found their way into successful treatment strategies, these findings illustrate how preclinical data are translated into novel treatment strategies. In addition, the underlying preclinical data may function like a ray of hope as they suggest that cellular abnormalities linked to the development of gastroparesis are potentially reversible.
One conceptual model envisions impaired opening of the pyloric channel as a cause for gastric retention, providing a rationale for treatments targeting this muscle. After two negative trials of botulinum toxin injection into the pylorus29,30, the potential role of pyloric dysfunction in gastroparesis has surfaced again in two forms. On the diagnostic side, the EndoFLIP (endolumenal functional lumen imaging probe) device allows detailed assessments of the pylorus’s active and passive mechanical properties, which seem to be altered in gastroparesis and appear to correlate with symptoms and emptying31,32. If this test is confirmed, we need to examine whether it enables us to identify a subgroup that could indeed benefit from interventions targeting this muscle structure. On the therapeutic side, per-oral endoscopic myotomy not only is feasible but also comes with impressive preliminary findings in early open-label trials33. In light of the similar early enthusiasm with botulinum toxin therapy, followed by disappointing results in randomized controlled studies29,30,34–37, it is essential to integrate more detailed functional assessments of this muscle (for example, by using the EndoFLIP) and to assess the true impact of this intervention with controlled trials.
Several other observations about treatment options and impact have emerged during the last decade. First, a consortium of tertiary referral centers completed longitudinal studies that tracked symptoms and findings in patients who received treatment at the various sites for gastroparesis. Though not standardized and thus limiting our ability to assess specific treatment approaches, management was largely driven by the very experts who set the tone in discussions about the diagnosis and treatment of this illness. The data seem to be disappointing as even in expert hands, symptoms largely remain stable with less than one third of the patients improving during follow-up16,23. As already indicated, the observational design of this cohort study does not allow conclusions about specific steps. Yet it is important to contrast this message with the results of randomized controlled trials completed recently. We compiled data from double-blinded placebo-controlled trials published within the last decade and examined response rates for dichotomized endpoints (Figure 1) or changes in severity ratings for continuous data (Figure 2). Virtually all showed only marginal or even no differences compared with placebo. However, placebo responses were very high across the board, and vomiting frequency dropped by as much as 70%, to cite just one example38. These results are difficult to reconcile with the largely stable symptoms reported by the consortium. In regard to one specific modality, gastric electrical stimulation, the benefit reported in retrospective case series and during the open-label trials, but not the blinded phase of controlled trials39–41, did not seem to translate into tangible changes for type 1 diabetics who often underwent device implantation as part of their management through members of the consortium16. Most drug trials focused on ghrelin agonists, and short- but not long-term studies of one agent showed benefit over placebo7,8,42,43. A phase II trial of relamorelin, another ghrelin agonist, did not demonstrate improvement in vomiting as the primary endpoint when compared with placebo, but the active intervention was superior in an analysis of some secondary endpoints38. After prior failures of motilin agonists4–6, another agent surfaced, again with promising results after a single dose in diabetics with gastroparesis44. More appropriate long-term studies will be needed to see whether the most recent introduction is indeed different from the previously tested agents with a similar mechanism. For now, the most convincing standard therapy for gastroparesis is dietary intervention45. Positive prognostic data, such as onset after an infectious prodrome, male gender, or antidepressant use, as well as negative prognostic factors, most notably opioid use and pain predominance23, may enable providers to risk-stratify and tailor treatment approach or intensity. For providers outside of tertiary referral centers, the high placebo response rates and comparable effects with approaches based on very different mechanisms of action have important implications. If numbers needed to treat do not differ dramatically between therapies, the number needed to harm (that is, the side effect profile) becomes more important. While the effects of antiemetics and other agents commonly used in gastroparesis have been less systematically studied, the controversies surrounding metoclopramide clearly highlight the relevance of this criterion in gastroparesis46–48.
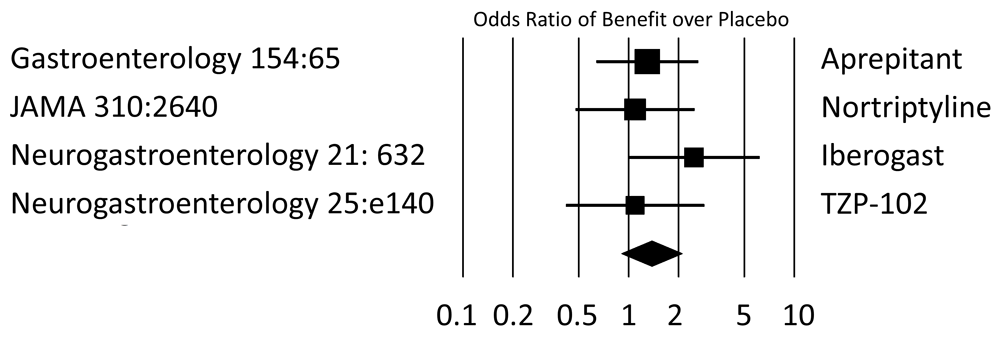
Trials published within the last decade and providing response rates were assessed to determine the odds of active therapy being superior to placebo. All but the ghrelin agonist TZP-102 used symptom scores; TZP-102 compared gastric-emptying rates which were dichotomized based on half-emptying times of less or more than 150 minutes. Only the herbal preparation Iberogast was superior to placebo when the primary endpoint was analyzed. Studies are labeled on the basis of the publishing journal with volume and first page. JAMA, Journal of the American Medical Association; Neurogastroenterology, Neurogastroenterology & Motility.
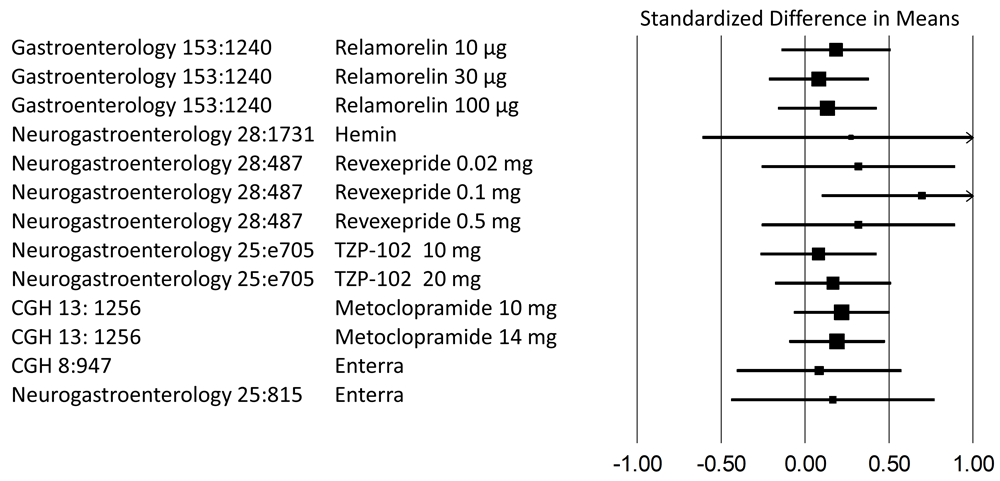
Placebo-controlled trials published within the last decade were analyzed to depict differences in symptom scores between active interventions and placebo. All data are based on normalized mean changes in symptom scores. As the primary outcome variable for studies on gastric electrical stimulation (Enterra) was given only as median, we chose an aggregate score based on symptom frequency. CGH, Clinical Gastroenterology and Hepatology; Neurogastroenterology, Neurogastroenterology & Motility.
With at least some of the novel treatments not meeting expectations, gastroparesis remains a difficult-to-treat disorder. However, there have been clear advances in our understanding of this and perhaps some of the other closely related diseases. In our short review, we have tried to point to potential areas for future studies. One key question, is whether we should see gastroparesis as a distinct disorder or whether it belongs in the spectrum of syndromes labeled as functional dyspepsia, as has been suggested49. Impaired motility clearly contributes to the pathophysiology of these common problems but includes more than just a delay in emptying. Thus, we need to assess whether other non-invasive tests, such as magnetic resonance imaging or single-photon emission tomography50,51, can provide a more comprehensive assessment of gastric motor function from accommodation to emptying and whether more detailed information allows more targeted and more effective therapy. If indeed emptying poorly correlates with symptoms and treatment effects, what can we offer beyond dietary advice? Recent studies give us some sense of direction, as anxiety and depression are associated with symptom severity10 and as antidepressant use functioned as a positive outcome predictor23. These results correspond to detailed mechanistic studies in functional dyspepsia that highlight the importance of affect52,53. Yet we still have to resolve some apparent contradictions, as well-designed trials led to inconsistent results with tricyclics not being superior to placebo in gastroparesis, while there was a benefit in a large cohort of patients with functional dyspepsia which included persons with impaired gastric emptying54,55. Going back to our call for a better and more comprehensive phenotypic characterization of patients, we may be able to identify subgroups with markers other than gastric emptying alone that may allow more targeted and more successful therapy. Concerns about metoclopramide led to a decreasing use of prokinetics with an apparent increase in antiemetics. Given the importance of nausea and vomiting as hallmark symptoms of gastroparesis, this development seems intuitively reasonable. However, the marginal benefit seen with aprepitant56 demonstrates the need for evidence rather than intuition. Most of our insight into the efficacy of antiemetics comes from the treatment of chemotherapy-induced nausea, which is typically an acute and only transient problem. Although the clinical manifestation may look identical, the underlying mechanisms will almost certainly differ, forcing us to better define the true benefit of these agents in gastroparesis. Perhaps most importantly, we need to develop approaches that will enable us to provide better prognostic information and treatment for patients where they are most commonly seen, outside the tertiary referral centers.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 08 May 18 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)