Keywords
Audiological rehabilitation, auditory plasticity, auditory training, childhood development, dichotic listening test, hidden hearing loss, right ear advantage, speech-in-noise recognition
Audiological rehabilitation, auditory plasticity, auditory training, childhood development, dichotic listening test, hidden hearing loss, right ear advantage, speech-in-noise recognition
Here we review some of the literature concerning the asymmetry of the human auditory system and use it to construct a model of how the central and peripheral components work together. The model uses the Doreen Kimura “structural model” of dichotic listening as its foundation [see Kimura, 1961; Kimura, 2011], and is augmented by the findings of the auditory scientists who have continued the work she began nearly 60 years ago.
The progress of audiological rehabilitation and auditory science research remains compromised by the continuing preoccupation with understanding hearing through knowledge of the peripheral hearing system [Hewitt, 2018; Musiek et al., 2017]. Only when the depth of our knowledge of the central auditory system matches that of the peripheral auditory system, and audiological practice is informed by the integration of this knowledge, can we expect to properly rehabilitate those with hearing problems.
There are ascending auditory pathways that connect both ears to both the left and right Auditory Cortices (ACs) (Figure 1). However, as Doreen Kimura discovered [Kimura, 1961; Kimura, 1967; Kimura, 2011], the primary connections are the crossed pathways. Normal hearing involves the right ear feeding sounds to the left AC and the left ear feeding sounds to the right AC.
In her research. Doreen Kimura made extensive use of various dichotic listening (DL) tests. One of the most popular dichotic listening tests is the Bergen dichotic listening test with consonant-vowel (CV) syllables (DL-CV) [Hugdahl & Andersson, 1986; Hugdahl & Asbjørnsen, 2013]. Each running of the Bergen DL-CV test involves 30 presentations using headphones. Each presentation involves the simultaneous playing of two randomly chosen CV syllables—one to the right ear and one to the left ear. The test uses only six CV syllables—all of them stop consonants combined with the /a/ vowel: /ba/, /da/, /ga/, /pa/, /ta/, /ka/. There are three DL-CV test runs, of 30 presentations each. The first run is the non-forced condition. This is then followed by the forced-right condition and then the forced-left condition. An option is to reverse the order of the forced conditions. Figure 2 includes the instructions given to test participants for the three different conditions.
For all three DL-CV test conditions, a correct report (%) is calculated for both the right ear and the left ear. Figure 3 shows the right and left ear scores that are typically found in DL-CV studies with normal hearing participants. It also includes the typical non-forced condition scores found for studies of participants who have had a callostomy—a surgical procedure involving some level of cutting of the corpus callosum so as to limit the spread of epileptic activity between the two hemispheres of the brain [see Musiek & Weihing, 2011].

Higher non-forced condition (NF) scores for the right ear than for the left ear. This difference increases with the forced-right condition (FR) scores and decreases with the forced-left condition (FL) scores. For those who have had a callostomy, the right ear has almost no competition from the left ear. (Data is contrived.)
The results for studies involving individuals with normal hearing typically show higher non-forced condition scores for the right ear than for the left ear. This difference increases with the forced-right condition scores and decreases with the forced-left condition scores. This many repeated set of findings has become known as the “right-ear advantage (REA)”. However, we should be careful to be more specific and refer to these findings as showing a “REA for CV syllables” (REA-CV). Doreen Kimura conducted many different dichotic listening studies [Kimura, 2011] and as well finding a “REA for digits”, also found a “left-ear advantage (LEA) for melodies” and a “LEA for non-verbal sounds”. Following on from this work Ley & Bryden [1982] found a “LEA for the emotional tone of speech”. The REA-CV finding is therefore only one of many dichotic listening study findings which demonstrate the asymmetry of the two hemispheres of the human brain and auditory system. The REA-CV is a result of the right ear being “directly” connected by the crossed pathway to the left AC, and the left AC being more specialised at phoneme processing. As the left ear is only “indirectly” connected to the left auditory cortex (via the contralateral pathway, the right AC and the corpus callosum) then it is at a disadvantage with a dichotic test involving CV syllables [Kimura, 2011]. By contrast, the left ear has the advantage with a dichotic test involving emotionally intoned sentences. This is because of the “direct” connection of the left ear to the right AC by the crossed pathway, and the right AC being more specialised at prosody processing. We can refer to this as the LEA-emotion or LEA-prosody.
Taken on their own, the non-forced condition scores for the REA-CV and the LEA-prosody reflect bottom-up processing advantages. However, the forced condition results (Figure 3) show that top-down driven attention to one of the ears is able to modulate the scores. In the case of attention to the right ear this equates to increasing the REA-CV, and in the case of attention to the left ear it equates to decreasing the REA-CV, and in some individuals even reversing the advantage altogether. Westerhausen & Hugdahl [2008] describe this as two components working together; one “in-built” and bottom-up that is stimulus driven, the other attention driven and top-down, that enables modulation of the “in-built” asymmetry. The Bergen DL-CV Test has been used by many different studies over many years. Kenneth Hugdahl and his team at Bergen University, Norway developed the test, and have used it as a research tool for over 30 years [Hugdahl et al., 2009; Westerhausen et al., 2015]. The Bergen Dichotic Listening Database [Westerhausen et al., 2015] and the resulting normative data provided with their test manual [Hugdahl & Asbjørnsen, 2013] enables an understanding of the effects of age upon the REA-CV. There has been little discussion of these age effects in the literature but they provide valuable insight into the lengthy maturity window of the human auditory system through childhood and adolescence, and also its gradual decline, beginning as early as the sixth decade (Figure 4).
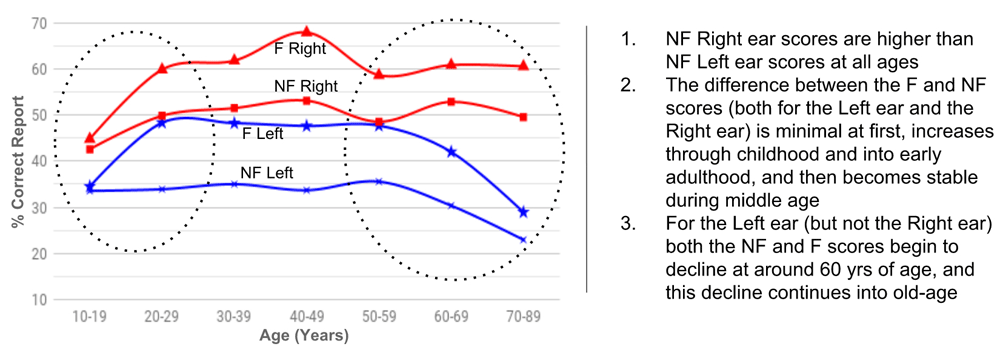
Normative data obtained from the ‘Bergen Dichotic Listening Test with CV-Syllables Manual’ [Hugdahl & Asbjørnsen, 2013].
Taking the effects listed in Figure 4 in turn:
1. The AC asymmetry seems to be “in-built” and lasts a lifetime
2. If we think of the ability to use top-down attention to modulate the in-built asymmetry of the auditory system as a skill, then it seems that this corticofugal skill does not begin to develop until the end of the first decade, but it then continues to develop into adulthood
3. There is evidence (see below) which shows that the interhemispheric pathways of the corpus callosum play significant roles in the deterioration of the left ear scores (and in the development of the above corticofugal skill)
A Finnish version of the Bergen DL-CV test was developed by researchers at the University of Turku, Finland and was used in their studies concerning childhood development [see Takio et al., 2009]. They too found that the top-down (corticofugal) skill to modulate the asymmetry of the auditory system does not begin to develop until the second decade. In a related study, this time in collaboration with Kenneth Hugdahl, they looked at the enhanced auditory processing skills of the congenitally and early blind [Hugdahl et al., 2004]. They found, as they predicted, that the blind subjects were significantly better than the seeing (control) subjects at using their top-down corticofugal skill when instructed to attend to the left ear stimulus. Using the REA-CV test, Hugdahl & Andersson [1987] found that, in a group of children aged from 8 to 9 years, there was a clear connection between increasing reading skills and increasing top-down corticofugal skills. In a related study, Andersson & Hugdahl [1987] found slower maturation of the corticofugal skill in a group of eight year old boys compared to a group of similarly aged girls.
Looking at the dichotic listening test results of adult individuals, rather than adult population averages, reveals that some test participants are able to entirely reverse the REA-CV when undertaking the forced left condition. Others are only able to increase their left ear score by small amounts, with the right ear score remaining considerably higher than the left ear score. The Bergen normative data [Hugdahl & Asbjørnsen, 2013] shows that there is a large between-individual variability. This leads to the proposition that, with some individuals, the skill to be able use top-down attention to modulate the asymmetry of the auditory system fails to properly develop.
An understanding as to why there is such a large between-individual variability is suggested by the dichotic listening test studies that looked at the differences between the DL-CV test scores of musicians and non-musicians. Milovanov et al. [2007] found that both choir-members and non-musical adults had similar REAs with the non-forced condition NF. The non-musical adults still had a clear right ear advantage with the forced left condition. The choir-members however, had a clear LEA with the forced left condition. The musicians had developed the skill to moderate the asymmetrical auditory system, while the non-musicians had not.
Several different auditory science research teams have conducted many different music related auditory studies in recent years. Most of these have concluded that with children (and young adults) there is a strong relationship between the extent of music practice and the enhancement of neural responses to speech, and as a consequence, better hearing speech-in-noise recognition
See, for example, the review paper of Strait & Kraus [2014]. These studies additionally found that the auditory training effects of music continued until the end of adolescence [see Krizman et al., 2015]. The REA-CV literature reveals the same finding, and in Figure 4 we can see that the difference between the forced and non-forced scores (both for the left ear and the right ear) is minimal at first, increases through adolescence and into early adulthood, and then becomes stable during adulthood. Tonal pitch processing is an important component of musical perception [Zatorre et al., 2002] and can be measured using the scalp-recorded frequency following response (FFR). Coffey et al. [2017a] found that the strength and fidelity of the FFR correlates well with speech-in-noise recognition scores. Also see Du et al. [2011]. They also found that the effect was stronger with the right AC than with the left AC. With young adults, Coffey et al. [2016] found a strong, right-asymmetric contribution to the FFR from the human auditory cortex, and that the magnitude of the response was related to musicianship. It is well accepted that musical training enhances abilities related to such things as pitch, rhythm and melody. However, there does remain some disagreement in the literature about the benefits of music experience skills with regards to improved recognition of speech-in-noise. Madsen et al. [2017], for example, found that although musicians showed better fundamental frequency discrimination, this did not translate into better speech-in-noise recognition in their study. However, in their review of the results of similar studies they did find examples of a clear speech-in-noise recognition benefit of musicianship. These particular studies differed from the others (and their own) in that speech-in-noise recognition was measured using a test that spatially separated target speech from noise.
The vast majority of the speech-in-noise studies in the literature describe their use of speech audiometry tests that use headphones. Such tests do not represent realistic listening situations, and do not test speech-in-noise skills that relate to the asymmetry of the auditory system [Coffey et al., 2017a; Hewitt, 2018]. Nevertheless, a recent review concerning the speech-in-noise recognition advantages of musical training was still able to conclude that, with 18 out of 20 studies showing an effect, musicians are better at hearing speech-in-noise [see Coffey et al., 2017b].
Several research studies concerning the connections between music and speech processing have focussed upon the top-down driven control of peripheral hearing by the medial olivocochlear (MOC) system, and the enhanced MOC systems of those who have undertaken musical training. Bidelman et al. [2017] found that musically trained individuals show enhanced ipsilateral and contralateral cochlear gain control, and that there is a correlation between MOC strength and length of training. Musical training strengthens dynamic MOC activity, and speech-in-noise recognition is described as a likely benefit of the enhanced corticofugal control of the peripheral hearing system shown by musicians [Perrot & Collet, 2014]. Using DL-CV testing, Markevych et al. [2011] found that those with greater MOC strength were better at using top-down corticofugal skill to overcome their REA-CV when instructed to attend to the Left Ear stimulus.
Recent studies made possible by non-invasive neurophysiological techniques have enabled closer inspection of the parallel processing by the two ACs during childhood. The evidence suggests that even though asymmetry is in-built, the asymmetry is relatively immature at birth, matures through childhood and adolescence, and is not mature until early adulthood.
Ari-Even Roth et al. [2016] found, as have many other otoacoustic emission based studies, that the transient-evoked otoacoustic emission results of a newborn hearing screening programme demonstrated that auditory system asymmetry is already apparent shortly after birth. Using event-related potential (ERP) techniques Musacchia et al. [2017] found that in the first year of life, early targeted acoustic experience can accelerate the maturation of both temporal and spectral processing. Using magnetoencephalography, Tang et al. [2016] showed that, compared to adults, the sound envelope following response was limited in capacity in children 3 to 5 years of age. Thompson et al. [2016] studied children 3–5 years of age to examine the evidence for temporal asymmetry early in life. They found a leftward asymmetry for higher-frequency oscillations and that this was more pronounced for those who scored higher on a speech-in-noise test. However, they did not find any rightward asymmetry for the lower-frequency oscillations. They proposed that this was a possible consequence of the known slower development of both white and grey matter structures in the right AC, and that this reflects the greater immaturity of the right AC at this young age. Clunies-Ross et al. [2018] studied temporal processing in children aged 7 and 9 years and, as expected, found evidence of left hemisphere specialisation (and a right hemisphere bias for spectral processing). They suggest that at this age, as the specialisation was not as prominent as with adults, the full extent of the asymmetry has yet to be reached, and that the immaturity of the right AC is greater. Using magnetoencephalography, Nora et al. [2017] studied children 6 to 8 years old and found that, compared to adults, the left hemisphere has yet to become dominant with regards (native language) phonological processing. They propose that at this age the learning of new words involves the use of the prosodic processing of the right hemisphere. Yathiraj & Vanaja [2015] showed that, in children between 6 and 10 years, different auditory processes matured at different rates. By measuring cortical responses, a study by Yamazaki et al. [2018] showed that, between the ages of 5 and 15 years, there is increasing specialisation of the two ACs. Using ERP, Mahajan & McArthur, [2013] studied left and right hemisphere auditory processing in adolescents aged between 10 and 18 years and found that they seem to mature at different rates, with the latter taking longer and continuing well into adolescence. In their longitudinal study using auditory brainstem response, Krizman et al. [2015] identified change taking place in auditory brainstem function between ages 14 and 17. They concluded that compared to the first decade different kinds of maturation take place in the second, and that the beginning of adolescence marks a transitional point.
We have described how human hearing develops throughout the first two decades of life and involves a slow but ever-increasing specialisation of the two auditory cortices. The related binaural skill of sound source localisation has also been found to gradually develop throughout childhood, adolescence and into early adulthood [see Freigang et al., 2015; Glyde et al., 2013; Grothe et al., 2010]. Spierer et al. [2009] studied brain-damaged patients and showed that auditory spatial representations for both left and right hemispaces are a function of the mature right hemisphere. At et al. [2011] reported the same finding in a study that used single pulse transcranial magnetic stimulation (to temporarily alter normal brain function). The Freigang et al. [2015] study includes a description of the age effects on sound localisation; with minimum audible angle (MAA) scores for 8–12 year olds not as mature as the adult-like MAA scores of 13–18 year olds. Glyde et al. [2013] describe how the many studies that have used the listening in spatialized noise-sentences test (LiSN-S) to measure the ability to process the sound localisation cues (measured by the LiSN-S “high-cue” score) have shown spatial processing continuing to mature until early adulthood.
The two decade maturation of AC specialisation and sound source localisation is prohibited by single-sided deafness (SSD). An understanding of SSD outcomes therefore enables consideration of the possible outcomes of a binaural auditory system that fails to fully mature. Zhang et al. [2018] found that unilateral hearing impairment lasting longer than 24 months drove cortical functional changes including enhanced interhemispheric AC connectivity and altered connectivity with visual and somatosensory networks. In a review, Eggermont [2017] concluded that, irrespective of the age of induction of a single-sided hearing loss, several hearing system adaptations take place, including modified interhemispheric connectivity and the near disappearance of the contralateral dominance of the ascending pathways. These adaptations take place over a few months and this short timescale can even mean that these adaptations will affect some individuals (both children and adults) who suffer from persistent otitis media with effusion (OME). Using REA-CV testing, Asbjørnsen et al. [2000] found that children who had undergone a myringotomy for persistent OME (at an earlier age) were less able to modulate the REA-CV advantage. Jafari et al. [2016] reported similar findings. Gordon et al. [2013] found that after 18 months of unilateral hearing with a cochlear implant that there was a loss of the contralateral dominance of the ascending auditory pathways and that this maladaptation still remained even after 3–4 years of subsequent use of bilateral cochlear implants (see also Polonenko et al. [2017]). Gordon et al. [2015] provide a summary of the hearing problems caused by SSD and some of the consequences. These include difficulty in hearing speech in noisy situations, impaired speech and language development, reduced verbal IQ, and the frequent outcomes of behavioural problems and requirement for individualized educational assistance.
In their review of the role of the corpus callosum in dichotic processing Musiek & Weihing [2011] concluded that dichotic listening scores relate to the physical development of the corpus callosum. Dichotic listening performance improves until adolescence matching the increasing size of the corpus callosum. In their review paper Westerhausen & Hugdahl [2008] describe how the corpus callosum plays a pivotal role in the central auditory system. Sammler et al. [2010] show that the posterior pathways of the corpus callosum are involved in the integration of the syntax processing of the left AC and the prosody processing of the right AC. In a review, Homae, [2014] concluded that during early years, the processing of auditory stimuli changed from independent ACs to mutually inhibitive ACs and modified corpus callosum interhemispheric connectivity. As Musiek & Weihing [2011] describe, some of the interhemispheric connections (between the two ACs) resulting from the maturation process are excitatory, others are inhibitory, and the maturation involves an increased rate of transfer of information (as a consequence of fibre myelination). In a review paper Bamiou et al. [2007] describe how the corpus callosum size increases until the third decade of life and then decreases again after the fourth decade. They also suggest that brain lateralization depends upon fast inter-hemispheric transfer, and that the corpus callosum has an important role in the processing of binaural cues and spatial hearing. In their recent review of the white matter asymmetries of the nervous system Ocklenburg et al. [2016] concluded that, although further investigation is still needed to understand the detail of the pathways and the inhibition and excitation involved, the hemispheric functional asymmetries depend upon the CC and its maturation path. The importance of the corpus callosum to auditory processing means that, as it “normally ages”, it also plays a pivotal role in what has become known as central presbycusis. The review by Musiek & Weihing [2011] reports that the worsening of left ear scores in dichotic listening tests begins in the middle of the sixth decade, as the corpus callosum begins to reduce in size (see also Figure 4).
We have learnt about how the peripheral auditory system and central auditory system work together by looking at dichotic listening literature and the auditory science literature. In the dichotic listening literature review we re-examined and re-interpreted the effects of age data, and then went on to look at the different DL-CV test scores of musicians and those with no sight. With the auditory science literature review we focussed upon the relationship between musical training and auditory processing skills, the enhanced corticofugal control of the individual cochlea by musicians, the slowly maturing specialisation of the two auditory cortices, and the pivotal role of the corpus callosum in the increasing AC asymmetry during the first two decades of life. Additionally, the SSD literature was examined to consider the possible outcomes of a binaural auditory system that fails to fully mature. The findings of this set of literature reviews are highly consistent and complementary, and have led to a set of conclusions about the human auditory system.
The human auditory system has evolved to be capable of performing the real-time processing of complex acoustical stimuli such as speech and music. Some individuals can even manage this task in the presence of competing background noises. With these individuals, it seems that they have specially trained their auditory system through more frequent binaural intensive and challenging listening experiences. This extensive training seems to result in increased asymmetry, increased neural enhancement, and greater corticofugal control of the individual cochlea. With greater maturity, the sense of hearing has been divided into two, with the left AC responsible for temporal processing and the right AC responsible for spectral processing [also see Sininger & Bhatara, 2012; Zatorre et al., 2002], and spatial representation. The processing of stop consonants for example requires millisecond differences to be detected and is a function of the left AC. The changing melody of musical notes occurs over seconds and its processing is a function of the right AC. When listening to speech-in-noise, these individuals are able to use parallel processing, with the heavy myelination of corpus callosum fibres enabling fast interhemispheric communication between the two ACs. To enable speech recognition in noise, right AC neuron populations (measured using the scalp-recorded frequency following response) are phase-locked onto the low-to-middle-frequency periodical acoustical features of the sound source [Du et al., 2011], sound source locations are more precise, and corticofugal control of OHCs is able to modulate cochlea sensitivity and to restore lost dynamic range [Perrot & Collet, 2014]. By contrast, the adult outcome for those who are limited to small numbers of binaural intensive listening experiences during the first two decades, is an untrained and immature auditory system. They are unable to use parallel processing, the phase locking to an individual sound source is imprecise, they have little corticofugal control of the OHCs, and they struggle to understand speech in the presence of background noise. In sum, while some asymmetry exists at birth, it is life experience that shapes the detail of the auditory processing capability and the functional asymmetry of an individual's auditory system.
The auditory system maturation window seems to be open from birth until the beginning of adulthood with the maturation taking place during the first decade relating more to the temporal processing specialisation of the left AC. The maturation taking place in the second decade appears to relate more to the right AC and its spectral processing and sound location specialisations. A significant outcome of the maturation during the second decade is the establishment, or not, of corticofugal control of the peripheral auditory system, via the descending efferent pathways. The review paper by Terreros & Delano [2015], as well as discussing the MOC control of the OHCs, describes the recent findings concerning the efferent control of the inner hair cells (IHCs) of the cochleae via the lateral olivocochlear (LOC) neurons. Their working model proposes that there are three afferent-efferent feedback loops that enable the dynamic corticofugal modulation of the IHCs and OHCs. They describe the LOC/MOC control of the IHCs/OHCs as a top-down frequency filter that facilitates, for example, speech recognition in noisy environments.
1. The mature auditory system is to some extent asymmetrical and with some individuals the two ACs are capable of parallel processing
2. With regards the processing of speech, the asymmetry equates to a left AC (right ear) responsible for phoneme processing and a right AC (left ear) responsible for prosody processing. Temporal (phoneme) processing mostly matures during the first decade of life, whereas spectral (prosody) processing takes longer to mature. The mature right AC is responsible for the neural representation of both the left and right hemispaces.
3. The more extensive and challenging the auditory listening experience leading up to adulthood, with regards the need to simultaneously process both temporal and spectral features, the greater the asymmetry, the greater the neural enhancement, and the more refined the auditory processing skills
4. Musical training is known to improve sensory representation, sequencing skills, working memory, auditory attention, stream segregation, and top-down expectations; resulting in “musicians” having superior speech perception in noise ability, compared with “non-musicians” [Chandrasekaran & Kraus, 2010]
5. Very different childhood/adolescent listening experiences mean that auditory processing capability and the extent of asymmetry varies considerably—between children, between adolescents, between young adults, and between adults. These differences are “completely hidden from” and not measurable using standard audiometry tools (Hewitt, 2018; Musiek et al., 2017)
6. With some individuals top-down attention is able to modulate auditory system asymmetry. This corticofugal “skill” is well-developed in some adults but hardly at all in others. It appears connected with the maturation of the prosody processing of the right AC and its development occurs (or not) during the second decade of life.
7. The corticofugal “skill” involves all of the auditory pathways. This includes the ascending afferent pathways, the two ACs, the interhemispheric pathways of the corpus callosum, the descending efferent pathways (including the olivocochlear efferent fibres), and ultimately the dynamic control of the hair cells of the two cochlea (see Figure 5).
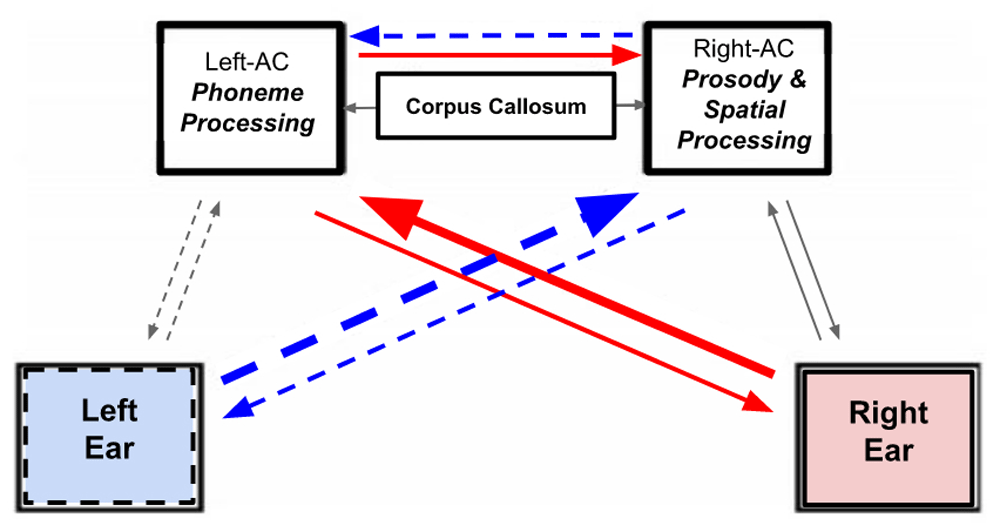
The right ear feeds sounds to the left auditory cortex (AC) and the left ear feeds sounds to the right AC, using the crossed ascending pathways. When listening to speech, the left AC is responsible for phoneme processing and the right AC is responsible for prosody and spatial processing. The corpus callosum interhemispheric pathways connect the two ACs together. Top-down attention can modulate the asymmetry and involves the descending (efferent) pathways and the dynamic control of the hair cells of the two cochlea.
The “structural model” of Doreen Kimura [Kimura, 1961; Kimura, 2011] was a precursor to the “asymmetric sampling in time” (AST) model [Poeppel, 2003]. The AST model equally aims to provide a framework for understanding the asymmetry of the function of the two ACs. It describes them as anatomically similar but functionally specialised, with the left AC adapted to extracting information from shorter (20–40 ms) time windows, and the right AC adapted to extracting information from longer (150–250 ms) time windows. In attempting to aid the understanding of the asymmetry of the human auditory system this article shares the objectives of the Doreen Kimura “structural model”, the David Poeppel AST model, and the series of Hugdahl et al. and Westerhausen et al. dichotic listening studies (Westerhausen & Hugdahl [2008] provide references to most of these). In a related review Tervaniemi & Hugdahl [2003] describe the lateralisation differences between the encoding of speech and the encoding of music. This article, however, has an objective over and above an updated review of our knowledge concerning the asymmetrical auditory system—that of translating the knowledge into the availability of improved rehabilitation in the audiology clinic.
The original articles of Kimura [1961]; Kimura [1967] and Poeppel [2003] have been cited thousands of times in the literature and yet, now nearly 60 years since the first of these articles, audiological rehabilitation continues to fail to recognize the existence of any asymmetry in the human auditory system [Hewitt, 2018; Musiek et al., 2017]. One important outcome concerns speech audiometry. With a lack of consideration of the binaural nature of hearing and the asymmetry of the central auditory system, most speech audiometry tools are unable to determine speech-in-noise recognition capabilities reliably [Hewitt, 2018]. Their lack of usefulness has meant little use in the audiology clinic and their use restricted to auditory science research laboratories. Most of these tests use headphones and “one ear” testing (see, for example, Hewitt [2008]). Such tests involve the perception of sound inside the head, do not represent realistic listening situations, do not involve spatial cues, do not test the speech-in-noise skills that relate to the asymmetry of the auditory system, and do not test prosody (e.g. emotion recognition) [Coffey et al., 2017a; Hewitt, 2018]. As a consequence there are limitations to the validity of the results and conclusions of the auditory science research studies that have used such “one ear” speech testing. This is the case with the majority of the hearing research published in the academic literature, with the validity of the results of those studies concerned with speech recognition in noise being more in question (see also Phatak et al. [2018]).
The model of the asymmetrical human auditory system (and those of the past) leads us to the conclusion that changes to the current practices of both audiologists and auditory science researchers are needed. The suggestions included in this and subsequent sections of this article are intended to stimulate discussion about the required nature of these changes.
Greater knowledge of how human hearing works improves our abilities to assess hearing, to diagnose hearing problems and to manage those with hearing difficulties. The model suggests, for example, that there is a need for:
1. Wider-scope hearing test tools (for the audiology clinic and auditory science laboratory) that involve the “exercising” of both cochlea, both auditory afferent pathways, both auditory cortices, the corpus callosum, and both auditory efferent pathways.
2. Hearing test tools that provide normative data for the lifespan (i.e. separate normative data for children, adolescents, young adults, adults, and old adults).
a. Not unlike height and weight, auditory system development during childhood requires close monitoring, with the appropriate interventions used when developmental problems are identified.
b. Auditory system aging (presbycusis) requires close monitoring, with the appropriate interventions used sooner rather than later (see also Glick & Sharma [2017]).
3. The development of auditory training modules (ATMs), for the lifespan (i.e. different ATMs for children, adolescents, young adults, adults, and old adults).
The simple model described in Figure 5 generates as many questions as it answers; the following are only some of these questions.
The model supports the use of ATMs for those with hearing problems based on the premise that “real life” hearing training for subsets of the population, such as musicians or the blind, bestows auditory skills such as speech recognition in noise. Assuming that practical (and perhaps personalisable) ATMs can be developed then there is a prospect that they will be able to play a significant role in:
The rehabilitation of cochlear implant recipients [see Anderson & Kraus, 2013]
The management of those suspected to have Auditory Processing Disorder [see Moore et al., 2018; Weihing et al., 2015]
Childhood Speech and Language development [see Clunies-Ross et al., 2018]
And perhaps they hold out the promise of:
Effective speech perception in noise for the majority of adults
Prolonging hearing skills into old-age [see Strait & Kraus, 2014]
Delaying the aging of the auditory system and cognitive decline [see Hewitt, 2017; Humes & Young, 2016; Livingston et al., 2017; Lin et al., 2013; Loughrey et al., 2018]
Can a form of dichotic listening test be developed that will reliably and efficiently predict speech recognition in noise ability? There is a case for using the existing REA-CV test as a screening tool. Also see Asbjørnsen et al. [2000] and Jafari et al. [2016].
Unfortunately, unlike with the DL-CV studies, the literature does not provide significant amounts of data from the use of dichotic listening-prosody testing. Would such data provide further insight into the slower development of the right AC?
It is well accepted that standard pure tone audiometry (PTA) is unsuitable for measuring speech in noise recognition capability [Musiek et al., 2018]. More recently it has been shown to be insensitive to cochlear synaptopathy (CS). CS can result from excessive noise and from aging. Standard PTA is unable to detect CS until it becomes extreme, and as a consequence the condition has become known as “hidden hearing loss” [Kujawa & Liberman, 2009; Liberman et al., 2016]. Ipsilateral (“one ear”) speech-in-noise testing was used by Liberman et al. [2016] to compare a group of people with normal sensitivities up to 16 kHz with a group of people with normal sensitivities up to 8 kHz but threshold elevation for the 10–16 kHz range. The latter showed worse speech-in-noise discrimination scores. The study concluded that to increase the possibility of CS detection, both high-frequency audiometry and speech recognition testing should be used in the audiology clinic with greater regularity. The model of the asymmetrical auditory system leads us to proposed extensions to the recommendations made by Liberman et al. [2016]. While the more commonly used speech audiometry tools are designed for ipsilateral presentation of speech and noise to a single ear using headphones, a small number of sound field speech audiometry tools are currently available that use loudspeakers for spatially separated speech and noise presentation. Although normative data is perhaps limited, such sound field speech audiometry tools enable some degree of measurement of actual binaural speech-in-noise recognition capability. Therefore:
To increase the sensitivity to hearing loss not detectable using standard PTA, high-frequency audiometry and sound field speech audiometry testing should be used in the audiology clinic with greater regularity.
To increase the sensitivity to speech-in-noise recognition capability, sound field speech audiometry testing should replace headphone based (“one ear”) speech audiometry in the auditory science laboratory.
Sound field speech audiometry additionally supports “before and after” hearing aid evaluation [Hewitt, 2018].
To improve speech-in-noise recognition outcomes when fitting hearing aids, less reliance should be placed upon the results of PTA scores, with more reliance placed upon “before and after” sound field speech audiometry results
Gordon et al. [2015] reached a similar conclusion: that standard paediatric audiology clinic test procedures require modification, as they fail to be sensitive to the increased risk of educational difficulties that result from single-sided hearing. See also Musiek et al. [2018].
This article extends a listening model first published nearly 60 years ago. The extended model describes a human auditory system that, while some asymmetry exists at birth, requires extensive and challenging binaural listening experiences leading up to adulthood to ensure the full maturation of the asymmetry and the associated speech-in-noise recognition capabilities.
Only when the depth of our knowledge of the central auditory system (CAS) matches that of the peripheral auditory system, and audiological practice is informed by the integration of this knowledge, can we expect to effectively rehabilitate those with hearing problems. While our knowledge of the CAS remains far from complete and this study and model make only a minor contribution in this respect, one clear conclusion has been arrived at—auditory science is compromised by a lack of “asymmetry sensitive” tools.
We have hitherto thought of hearing as nature. We now know that plasticity is a defining feature of the auditory system during its maturation, middle age, and the years of presbycusis. We need to think more seriously about how best the auditory system can be nurtured.
No data is associated with this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Are arguments sufficiently supported by evidence from the published literature?
Partly
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Partly
References
1. Iliadou V, Weihing J, Chermak G, Bamiou D: Otoacoustic emission suppression in children diagnosed with central auditory processing disorder and speech in noise perception deficits. International Journal of Pediatric Otorhinolaryngology. 2018; 111: 39-46 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Auditory Processing Disorder
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 29 May 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)