Keywords
Lactation, Breastfeeding, Reference ranges, Reference limits, Normal function, Maternal, Breastmilk
Lactation, Breastfeeding, Reference ranges, Reference limits, Normal function, Maternal, Breastmilk
The rationale for writing a review is usually based on bringing together recent major advances and published discussion in a particular area of research. Unfortunately, basic research into the physiology and biochemistry of the lactating human mammary gland is limited, and there have been no major advances toward the assessment of its normal function in recent times. As a result, the lactating mammary gland is a poor cousin when compared with other major organs such as the heart, brain, liver, lungs, and kidneys. These all have an array of objective tests available to assess function. This review examines the evidence available toward the development of methods for the objective assessment of lactation. Discussion will be limited to the maternal aspects of lactation physiology and biochemistry during the period of exclusive breastfeeding of the infant (that is, the period of time from birth and during the period of exclusive breastfeeding).
The mean milk production of lactating women by 8 days postpartum (Figure 1) is 650 mL/24 hours, and from 1 to 6 months of lactation the mean range for exclusively breastfed infants is between 750 and 800 mL/24 hours1. Therefore, for mothers exclusively breastfeeding their babies, it can be calculated that the energy output in human milk accounts for about 20% to 30% of the maternal resting energy requirement2. This large energy commitment clearly demonstrates the evolutionary importance of lactation for human survival. The World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of an infant’s life followed by the addition of nutritionally adequate and safe complementary foods while breastfeeding continues for up to 2 years of age or beyond3. In Australia, there is a high rate of lactation initiation (96%). However, these figures decline rapidly after birth, and only 15.4% of infants are exclusively breastfed at 5 months of age4. Thus, in Australia, as in many other high-income countries, almost all mothers elect to breastfeed, but many encounter difficulties that affect their ability to continue.
Vital to the assessment of function of major organs is the objective measurement of various aspects of their physiology and biochemistry and the comparison of these values with reference ranges. An objective measurement of normal function can then form the scientific basis for identification of potential abnormality (function falling outside the normal range), diagnosis of disease, and the evaluation of treatments. Unfortunately, there are no routine clinical tests available for the assessment of normal function for lactation.
In order to identify objective measurements for normal lactation function, it is first necessary to define normal lactation. Here, “normality” is considered in the biological sense, namely that normal function occurs naturally and not as a result of any disease, treatment, or genetic abnormality. Normal function does not require medical intervention or support. Thus, the following definition for lactation during the first 6 months after a term birth is proposed.
Normal human lactation
In the context of biological function, a reference range describes a range of values for a physiological measurement in a healthy person. A reference range for a particular measurement is the interval between which 95% of values for a reference group taken from the general population fall. Values outside the upper and lower limits of a reference range are not necessarily abnormal but can be considered to be indicators of possible pathology. A reference range is derived from a normal curve based on measurements for at least 120 healthy individuals. A reference limit describes cases where only one side of the range is of interest15.
The above definition of normal human lactation defines the inclusive and exclusive parameters for studies aimed at determining reference ranges or reference limits (or both) for the assessment of human lactation. With the exception of the growth rate of breastfed infants (WHO growth charts16), there are no studies that meet these criteria. Most substrates, metabolites, and hormones associated with human lactation change markedly according to the period of lactation, time of day, time of breastfeeding, duration of the breastfeed, and the degree of fullness of the breast. Thus, these factors need to be standardized for the development of normal ranges across the course of lactation. These deficiencies notwithstanding, it is nevertheless important to review the literature and construct approximate objective parameters for human lactation.
This review concentrates on areas where even roughly defined reference ranges could greatly assist in the understanding of lactation function (and consequently dysfunction). In this context, maternal parameters associated with secretory activation17 and established lactation will be highlighted. In addition to this restriction to a consideration of maternal factors in the first instance, a pragmatic approach was taken to emphasize methods that could be implemented now (for example, blood progesterone and milk sodium) and then methods that could be readily developed in the future for clinical use (for example, milk lactose and milk production). There are many other factors that potentially could be used to assist clinical diagnosis, including certain proteins (α-lactalbumin), carbohydrates (oligosaccharides), lipids (medium-chain fatty acids), and enzymes (aurora kinase-A), but there is not enough evidence in human lactation to provide potential diagnosis and treatment protocols.
The breast reaches a mature functional state only during lactation18. The lactation cycle begins with conception. Pregnancy induces ductal proliferation and subsequent lobular alveolar development in the mammary gland. Alveolar development leads to secretory differentiation17 with the maturation of lactocytes and the production of unique milk components. Delivery of the placenta triggers secretory activation and the transition to copious milk secretion. Sustained milk synthesis requires the continuation of efficient and regular milk removal by the infant in the context of normal function or by breast pump or hand expression if required. The lactation cycle is completed when the breast returns to quiescence following weaning of the infant.
Lactation is established in two phases. First, secretory differentiation is observed as the breast develops the capacity to synthesize unique milk products in colostrum, including lactose, casein, α-lactalbumin, and lactoferrin. The second phase, secretory activation, begins around 60 hours (range of 24–72 hours) after birth and is triggered by delivery of the placenta19,20.
There is little information on variation in breast growth and function during pregnancy. Breast volume generally increases after conception, but there is considerable variation between mothers21. Importantly, this variation does not appear to influence the potential for milk synthesis postpartum5,19,22.
Secretory differentiation occurs from about 20 weeks of pregnancy and requires the action of a lactogenic complex of hormones. The increasing concentration of prolactin in maternal blood is related to the increased excretion of lactose in urine, and the increase in the concentration of human placental lactogen is related to breast growth. During this phase, the tight junctions between lactocytes are leaky, allowing milk constituents to pass into the blood. Lactose cannot be metabolized once it enters the blood vascular system and is excreted in the urine. Therefore, the 24-hour output of lactose in urine provides a measure of the synthesis of lactose in the mammary glands. Coupling the 24-hour output of lactose in urine with the measurement of lactose in colostrum provides an estimate of the rate of synthesis of colostrum during pregnancy.
These measurements confirm that the rate of colostrum synthesis at this time is low (about 30 mL/24 hours)17,23.
Secretory activation is a process manifest by the initiation of copious milk secretion (Figure 1). The increase in milk secretion is accompanied by many metabolic changes. The most documented of these changes are decreases in blood progesterone (Figure 2), milk protein, and sodium (Figure 3) and increases in milk lactose and citrate (Figure 4).
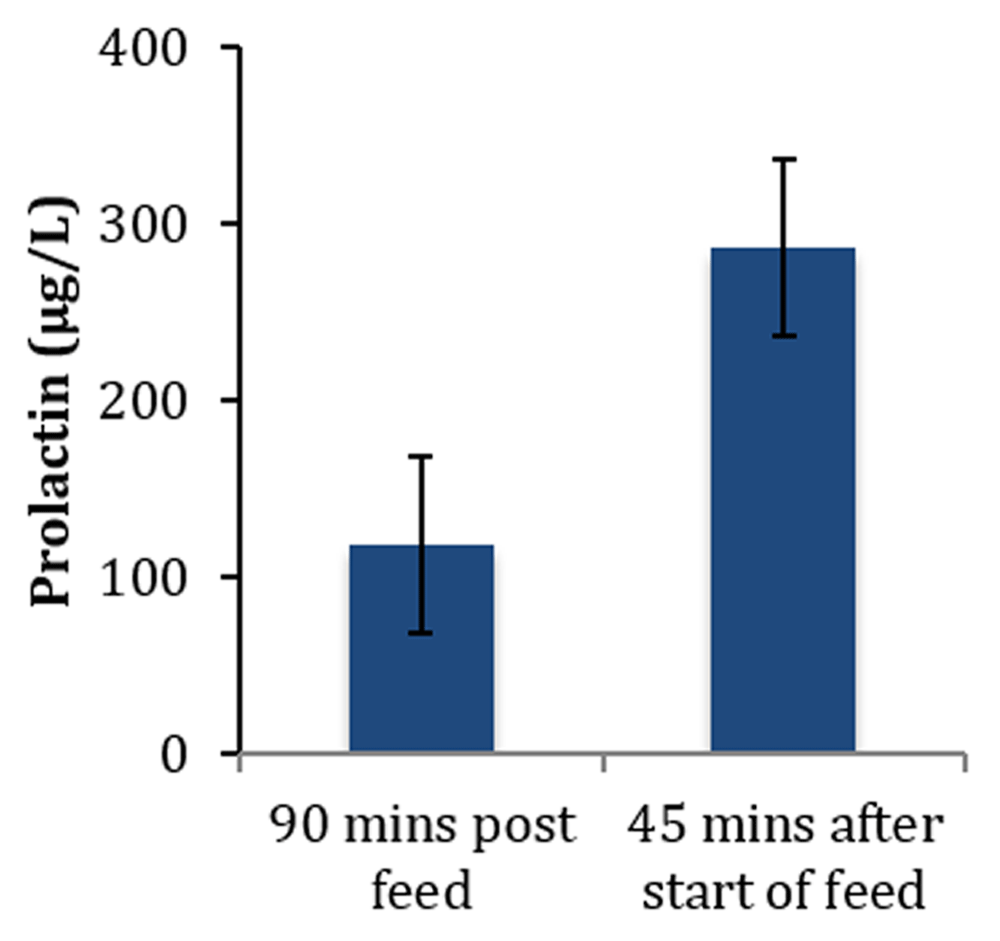
Data source: 32.
Colostrum is available to the infant for the first 60 hours (range of 24–72 hours) after birth19,20. The volume ingested by healthy newborns in the first 24 hours of life is small and mirrors synthesis (29 mL ± 24, mean ± standard deviation [SD]) (Figure 1). Research interest in colostrum is limited for two reasons: first, from the generalized lack of research into human lactation (see above) and, second, by its appearance and small volume. More than 300 years ago, Cadogan observed that, “When a child is first born, there seems to be no provision at all made for it: for mother’s milk seldom comes in ’till the third day: so that according to nature, a child would be left a day and a half or two days, without food; to me a very sufficient proof that it wants none”33. Furthermore, Dettwyler reported that, “In historical times, and even today, babies in some societies are denied colostrum, with all its beneficial properties, in the belief that it is a poisonous substance dangerous for the newborn”34.
In contrast to historical beliefs, evidence is now emerging that the first 3 days postpartum are critically important for the establishment of lactation. In a study of pump-dependent mothers who had delivered preterm, Meier et al. compared milk expression by using the standard suction pattern on a breast pump with using a pattern that mimicked the infant sucking35. The pattern was applied from birth to 3 days postpartum and then a standard pump was used up to 14 days postpartum. This treatment resulted in a 60% increase in milk synthesis from 6 to 14 days postpartum compared with the standard pumping pattern. Morton et al. studied pump-dependent mothers who had delivered preterm and combined hand massage with electric pumping in the immediate postpartum period and found that this intervention increased milk synthesis at 2 weeks compared with the use of a standard breast pump from birth51. Additionally, a study in the Democratic Republic of the Congo reported that 14% of mothers provided with standard care and 16% provided with the Ten Steps to Successful Breastfeeding program were exclusively breastfeeding at 6 months postpartum but that 45% of mothers provided with only steps 1 to 9 were exclusively breastfeeding at 6 months52,53. These findings show that subtle interventions in the early postpartum period can induce profound outcomes during established lactation.
Kuhn first demonstrated that the withdrawal of progesterone was related to the ovary switching from progesterone to 20αOH-progesterone synthesis54. The resulting fall in progesterone was shown to be the “lactogenic trigger” in the rat54. Subsequently, in all mammals studied, progesterone withdrawal has been found to be the trigger for secretory activation. In women, progesterone is synthesized in the placenta and falls precipitously by more than an order of magnitude after the delivery of the placenta (Figure 2). Consequently, colostrum synthesis continues until about 24 to 72 hours after birth when the process of secretory activation initiates the transition of colostrum into mature breastmilk. Thus, if viable fragments of placenta are retained after delivery, secretory activation will be either fully or partly inhibited.
Assays for progesterone concentration in blood are readily available. In addition, laboratory assays for progesterone concentrations in milk are available, and an in-home assay for urinary metabolites of progesterone has been developed55. Despite the availability of these assays, progesterone is never measured when assessing the initiation of lactation in women who are at risk of or appear to have impaired secretory activation.
Given what is known about progesterone withdrawal triggering secretory activation, it can be hypothesized that elevated blood progesterone is a sensitive indicator of retained viable placental fragments. Furthermore, it is possible that the inhibitory effects of elevated blood progesterone on secretory activation are reduced by the administration of mifepristone (RU486), a progesterone antagonist. However, more research is required before these possibilities can be adopted clinically.
At secretory activation, elevated concentrations of prolactin are observed during the period of progesterone withdrawal (Figure 2). Plasma prolactin is high early in lactation and progressively decreases, but levels at 6 months of lactation are still higher than those reported for non-lactating women32. The role of prolactin during the initiation of lactation is complex18. The concentrations reported for prolactin during the early postpartum period are highly variable and difficult to interpret. This can be attributed to the secretion of prolactin varying in response to the suckling stimulus, and peak values are observed about 45 minutes after the infant latches to the breast (Figure 3). Levels then decrease to about half the peak levels by the next breastfeed. Prolactin also shows a circadian variation56 and increases at mealtimes. No studies have monitored prolactin levels in relation to the timing of breastfeeds, meals, and time of day, and this probably accounts for the large variation in reported concentrations of prolactin during secretory activation. Prolactin is present as different isoforms and undergoes considerable post-translational modifications, including glycosylation, phosphorylation, proteolytic cleavage, and polymerization. These modifications influence the function of this complex hormone. It is clear that prolactin is required for successful secretory activation57, but it probably does not play a rate-limiting role.
The threshold concentration of prolactin (reference limit for normality) needed for normal secretory activation is unknown. This information is urgently required to ensure that mothers with normal concentrations of prolactin are not subjected to unwarranted medication with peripherally selective dopamine D2 receptor antagonist activity to treat low milk supply.
Closure of the tight junctions between lactocytes is related to the withdrawal of progesterone. At this time, the concentration of sodium in the mammary secretion rapidly decreases (Figure 5) as milk production increases (Figure 1) and provides a simple objective assessment of the progress of secretory activation (Figure 4)58. Indeed, sodium-sensitive electrodes that permit the measurement of sodium using only a few drops of breastmilk are available (Na+ LAQUA twin electrode, Horiba Scientific, Kyoto, Japan).
Milk volume and the concentrations of sodium, lactose, citrate, and total protein all appear to reach stable levels by 8 days postpartum (Figure 1, Figure 4, and Figure 5).
Decreases in sodium and total protein and increases in lactose and citrate in the mammary secretion can be used to monitor secretory activation5,59 (Figure 4 and Figure 5). Collation of the published values for the changes in progesterone, sodium, total protein, lactose, and citrate enables the calculation of weighted daily means. From these means and SDs, it is possible to calculate reference limits for normality (Table 1). It follows that the mean plus two SDs for progesterone in maternal blood and sodium and total protein in milk as well as the mean minus two SDs for lactose and citrate in milk provide reference limits that could be considered normal. Reference limits for days 3 and 6 are presented in Table 1. However, it must be recognized that these are tentative values because very few studies have determined the changes in blood progesterone and milk composition in the same mothers and, more importantly, most studies do not provide an objective assessment of the success of the mothers in establishing normal lactation.
| 3 days postpartum | 6 days postpartum | |
|---|---|---|
| Maternal serum | Maternal serum | |
| Progesterone, μg/L | <7.1 | <2.2 |
| Milk | Milk | |
| Total protein, g/L | <34.1 | <24.3 |
| Sodium, g/L | <0.81 | <0.84 |
| Lactose, g/L | >43.4 | >53.6 |
| Citrate, g/L | >0.24 | >0.92 |
As stated, milk sodium can be measured to track the progress of secretory activation. In the future, timed urinary lactose measurements after birth would also be useful. High levels of urinary lactose combined with low levels of milk sodium would indicate that lactose synthesis (milk synthesis) is occurring but the milk is not being removed from the mammary gland; that is, there is a “baby” problem rather than a “mother” problem.
Citrate has been claimed to be the harbinger of lactation60, but the metabolic stimulus driving the increase of this metabolite is not known. Citrate still provides a very good marker for secretory activation (Figure 5). It is not measured routinely in clinical pathology laboratories and therefore is not available at this time for the assessment of secretory activation.
It is important to define the difference between milk synthesis and milk production measurements. Whereas milk synthesis is a measure of the maternal capacity to synthesize milk, milk production is a measure of the infant’s ability to remove milk from the mother’s breasts (Figure 1). Daily progressive measurements of both of these parameters would be very useful in the assessment of lactation6,7. Twenty-four-hour measurements of milk production by weighing the infant immediately before and after each breastfeed to determine the milk intake is extremely difficult to carry out in the immediate postpartum period. Milk synthesis cannot currently be measured directly; however, the increase in milk citrate appears to be closely related to the mother’s ability to synthesize milk. A greater knowledge of the metabolic factors related to the increase in citrate in early postpartum milk secretion is required to confirm this possibility.
Once lactation is established from about 2 weeks postpartum, milk production remains relatively constant up to 6 months of lactation for infants that are exclusively breastfed1. Milk synthesis is not limited by the capacity of the mother to synthesize milk but rather by the infant’s appetite67. Thus, milk production in breastfeeding mothers is not a measure of the mother’s capacity to synthesize breastmilk but rather a measurement of the infant’s appetite. Furthermore, the variation in milk production reported between infants is large, ranging from about 500 to 1,000 mL/24 hours with a mean of 750–800 mL/24 hours1. The WHO Child Growth Standards provide reference values for breastfed infants; however, nearly all studies of normal intake of exclusively breastfeeding infants precede their release. Therefore, reference values for breastmilk production require further investigation. Nevertheless, measurement of milk production is currently the best objective method available to measure normal breast function during established lactation.
The most clinically feasible measure of breastmilk transfer to the infant is to weigh the infant immediately before and after each breastfeed over a 24-hour period67. From a nutritional standpoint, a longer period would be desirable; however, this method is quite demanding of mother and infant and the quality of the data degrades as the collection period is extended. Alternatively, each breast can be expressed for 10 minutes every hour for three consecutive hours in a calm environment. The volume of milk expressed at the third expression multiplied by 24 also provides an estimate of 24-hour milk synthesis67. It is possible to use this method in a clinical setting, but further validation is required. Total breastmilk transfer from both breasts over a period of 14 days can be measured by using the deuterium oxide dose-to-mother technique. This is an excellent technique for nutritional studies for determining macro- and micro-nutrient intake. However, this method is based on the dilution of the isotope over a period of 14 days and requires stable milk production over this period. Measurement of milk production is a good starting point for assessment of breast function, particularly when there are concerns that supply is inadequate.
Fat is the most variable component of human milk, and around 70% of this variation is due to the extent of breast fullness68. As the breast empties, the fat content increases68. This provides one of the few objective tests available to determine the proportion of available milk removed by the infant during a breastfeed. Mature milk from a full breast appears bluish-white because of the low fat content pre-feed. Post-feed, the milk changes in color to creamy white as the breast is drained and the fat content increases. This response is more obvious if the milk is allowed to settle. If there is not much change in the color of the milk between the pre-feed and post-feed samples, the baby has not removed much milk. Furthermore, if the pre-feed milk is bluish-white, the breast is full of milk, and if the pre-feed milk is creamy white, the breast is drained of milk. Because visual assessments of a breastfeed are very unreliable, observations of the color of the milk can provide only limited objectivity in the clinical assessment of a breastfeed. A more objective measure of breastmilk transfer was discussed previously. The change in fat content can be measured objectively with a creamatocrit centrifuge (Medela AG, Baar, Switzerland) (Table 2)69.
Milk ejection occurs because of a neuro-hormonal reflex triggered by stimulation of the nipple areolar area, which results in the contraction of myoepithelial cells surrounding the alveoli and subsequent expulsion of milk. It is a conditioned reflex and is responsive to environmental inputs. Each woman has multiple milk ejections during a breastfeed, but most mothers sense only the first. Milk ejection can be readily measured by ultrasound imaging of the non-suckled breast70. In mothers who do not sense milk ejection, measurement of an increase in duct diameter during a breastfeed can be used to confirm milk ejection.
In a whole of body comparison, the lactating human breast rivals the brain in its energy requirements and has the highest energy requirement of the organs in the reproductive cycle. Evidence-based reference ranges or limits for normal function (or both) provide a foundation for clinical diagnosis and treatment of problems encountered. Despite its metabolic importance, there is an enormous gap in our understanding of these parameters for the biochemistry and physiology of lactation. This review has shown that only approximate reference ranges can be calculated. Nevertheless, they provide a starting point.
There have been many successful health programs aimed at encouraging women to breastfeed their babies, but there is little ability to adequately monitor and support lactation initiation and establishment with objective tests. Under these conditions, it is not surprising that in high-income countries, even when most women choose to breastfeed, the rate of sustained lactation rapidly declines after birth. It seems obvious that to effectively “close the gap” more scientific evidence must be integrated with psychological and practical support for all women who want to breastfeed their infants.
Melinda Boss contributed conceptualization, preparation of the original draft, and review and editing of the manuscript. Hazel Gardner contributed data curation, formal analysis, and visualization. Peter Hartmann contributed conceptualization, supervision, and review and editing of the manuscript.
The authors declare that no grants were involved in supporting this work. Melinda Boss gratefully acknowledges research funding from the Family Larsson-Rosenquist Foundation (PG 52000600). Hazel Gardner is supported by an unrestricted research grant from Medela AG, Switzerland (PG52085000). Peter Hartmann holds an emeritus professorial position and declares that no grants were involved in supporting his contribution to this work.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 20 Jun 18 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Laurie A. Nommsen-Rivers, Associate Professor ... Continue reading Normal Human Lactation: Closing the Gap, is groundbreaking and sure to spark healthy debate on the development of globally relevant parameters for defining normal lactation.
Laurie A. Nommsen-Rivers, Associate Professor and Ruth Rosevear Chair of Maternal and Child Nutrition, University of Cincinnati, Cincinnati, Ohio
Icie Gertrude Macy was a pioneer in the field of milk biochemistry; she published the first comprehensive collection of known research on the composition of human milk in 1953 (1). Thirty years later a new generation of visionaries organized a conference specifically focused on human lactation methodologies in Winter Park, Colorado (2). From this conference, the classic text, Human Lactation: Milk Components and Methodologies, was published in 1985 and endures as a resource for investigators in human lactation. The Winter Park conference also served as fertilizer for what became the International Society for Research in Human Milk and Lactation (ISRHML).
Here we are more than 30 years since Winter Park and lacking an update specific to the components and methodologies of human lactation. After so many years, Normal Human Lactation: Closing the Gap, authored by Boss, Gardner, and Hartmann, is a groundbreaking contribution to human lactation research by an outstanding research team.
Like any trailblazing undertaking, the devil is in the details. I applaud Boss, et al., for publishing their critical review in an open forum. It is in this spirit that I respectfully posit that the greatest benefit in publishing Normal Human Lactation: Closing the Gap, is its potential to generate a consensus document by the current generation of lactation scientists on the parameters of “normal lactation.” My commentary focuses on three themes that exemplify the need for further input by a committee of lactation experts: 1) the parameters that define “normal” human lactation, 2) the selection of diagnostic indicators we use to evaluate abnormal lactation, and 3) in setting diagnostic values for normal lactation, consideration of human lactation reference values versus human lactation standards (a.k.a. global reference values).
Addressing the first theme, the authors eloquently make a case for establishing a clinical definition of normal function for lactation:
- An objective measurement of normal function can then form the scientific basis for identification of potential abnormality (function falling outside of normal range), diagnosis of disease and the evaluation of treatments. Unfortunately, there are no tests available for the assessment of normal function for lactation.
However, defining “normal” poses many challenges. For example, the first criterion listed by Boss, et al. for normal lactation is “comfortable for both mother and infant.”Is this statement perhaps conflating “normal lactation” with “normal breastfeeding?” In their introduction the authors specify that the review is …limited to maternal aspects of lactation physiology and biochemistry. This commenter supports their approach as it enables focus on one aspect of the breastfeeding dyad at a time. A mother may be able to lactate normally (i.e., produce milk of sufficient quality and quantity, however one defines “sufficient”), and even have normal external anatomy, but even so, there may be barriers to the mother-infant dyad being able to breastfeed comfortably. One of the most frequent reasons for the latter is a newborn with a tight lingual frenulum. Furthermore, normal physiology sometimes includes discomfort. Childbirth is an obvious example, but as any experienced breastfeeding mother can attest to, normal lactation can certainly be uncomfortable at times (e.g., a strong milk ejection reflex, or breast overfullness during phases of milk supply recalibration to infant demand).
Boss, et al., further defines normal lactation as the production of milk that supports “optimal growth and development.” This is indeed an important dimension of normal lactation. Typically, when growth of the exclusively breastfed infant is faltering, the clinician will consider those factors that impact caloric intake first and foremost (adequate frequency and thoroughness of breast emptying, adequate maternal milk volume, and rarely, lipid concentration). All of these angles are reviewed in Normal Human Lactation: Closing the Gap. An emerging area of consideration are the maternal diet-dependent micronutrients in human milk. While reasonably beyond the scope of the current review, vitamin and mineral composition of human milk is an important dimension of milk that supports “optimal growth and development. Notably, others are now addressing this important topic (3).
The above considerations may seem like splitting hairs, but these are the details needed on parameters that define normal lactation.
Addressing diagnostics, Boss, Gardner, and Hartmann, provide strong rationale for several of the proposed indicators of normal function, including maternal plasma hormone levels and milk biochemistry across the progression of lactation. A key diagnostic that was not covered in their review is sufficiency of mammary glandular tissue. With a diagnosis of insufficient milk production, the “triple feeding” process for increasing milk output requires extraordinary commitment on the part of a new mother (every 2-3 hours, breastfeed, followed by breast pumping, followed by supplemental feeds of infant formula to the newborn). A diagnostic test for evaluating glandular tissue sufficiency would go a long way towards facilitating an individualized and sustainable breastfeeding management plan for affected mothers.
The use of reference values versus standards is the third example of the need for further consensus on defining normal lactation. The authors do rightly provide the WHO Child Growth Standards (4) as a frame of reference for defining the production of milk that supports “optimal growth and development.” Notably, the Child Growth Standards were explicitly designed to be just that—standards. The WHO team was very intentional in differentiating the 2003 standards on how children should grow from previous child growth reference values which simply depict what is occurring in a given place at a given time.
The authors rightly declare that the reference values provided in this review are a starting point of values available today. It is very exciting to look to the future as our community of lactation scientists hone in on the outcome measures, timing, and selection criteria, for sampling milk volume and composition across the stages of lactation in mothers across the globe with “normal lactation.” It took a 3-day conference with much belabored contributions by over 50 authors to produce Human Lactation: Milk Components and Methodologies, and surely consensus on globally relevant reference values for normal human lactation will likely take a similarly collaborative effort. ISRHML…this is your call to action!
References
Laurie A. Nommsen-Rivers, Associate Professor and Ruth Rosevear Chair of Maternal and Child Nutrition, University of Cincinnati, Cincinnati, Ohio
Icie Gertrude Macy was a pioneer in the field of milk biochemistry; she published the first comprehensive collection of known research on the composition of human milk in 1953 (1). Thirty years later a new generation of visionaries organized a conference specifically focused on human lactation methodologies in Winter Park, Colorado (2). From this conference, the classic text, Human Lactation: Milk Components and Methodologies, was published in 1985 and endures as a resource for investigators in human lactation. The Winter Park conference also served as fertilizer for what became the International Society for Research in Human Milk and Lactation (ISRHML).
Here we are more than 30 years since Winter Park and lacking an update specific to the components and methodologies of human lactation. After so many years, Normal Human Lactation: Closing the Gap, authored by Boss, Gardner, and Hartmann, is a groundbreaking contribution to human lactation research by an outstanding research team.
Like any trailblazing undertaking, the devil is in the details. I applaud Boss, et al., for publishing their critical review in an open forum. It is in this spirit that I respectfully posit that the greatest benefit in publishing Normal Human Lactation: Closing the Gap, is its potential to generate a consensus document by the current generation of lactation scientists on the parameters of “normal lactation.” My commentary focuses on three themes that exemplify the need for further input by a committee of lactation experts: 1) the parameters that define “normal” human lactation, 2) the selection of diagnostic indicators we use to evaluate abnormal lactation, and 3) in setting diagnostic values for normal lactation, consideration of human lactation reference values versus human lactation standards (a.k.a. global reference values).
Addressing the first theme, the authors eloquently make a case for establishing a clinical definition of normal function for lactation:
- An objective measurement of normal function can then form the scientific basis for identification of potential abnormality (function falling outside of normal range), diagnosis of disease and the evaluation of treatments. Unfortunately, there are no tests available for the assessment of normal function for lactation.
However, defining “normal” poses many challenges. For example, the first criterion listed by Boss, et al. for normal lactation is “comfortable for both mother and infant.”Is this statement perhaps conflating “normal lactation” with “normal breastfeeding?” In their introduction the authors specify that the review is …limited to maternal aspects of lactation physiology and biochemistry. This commenter supports their approach as it enables focus on one aspect of the breastfeeding dyad at a time. A mother may be able to lactate normally (i.e., produce milk of sufficient quality and quantity, however one defines “sufficient”), and even have normal external anatomy, but even so, there may be barriers to the mother-infant dyad being able to breastfeed comfortably. One of the most frequent reasons for the latter is a newborn with a tight lingual frenulum. Furthermore, normal physiology sometimes includes discomfort. Childbirth is an obvious example, but as any experienced breastfeeding mother can attest to, normal lactation can certainly be uncomfortable at times (e.g., a strong milk ejection reflex, or breast overfullness during phases of milk supply recalibration to infant demand).
Boss, et al., further defines normal lactation as the production of milk that supports “optimal growth and development.” This is indeed an important dimension of normal lactation. Typically, when growth of the exclusively breastfed infant is faltering, the clinician will consider those factors that impact caloric intake first and foremost (adequate frequency and thoroughness of breast emptying, adequate maternal milk volume, and rarely, lipid concentration). All of these angles are reviewed in Normal Human Lactation: Closing the Gap. An emerging area of consideration are the maternal diet-dependent micronutrients in human milk. While reasonably beyond the scope of the current review, vitamin and mineral composition of human milk is an important dimension of milk that supports “optimal growth and development. Notably, others are now addressing this important topic (3).
The above considerations may seem like splitting hairs, but these are the details needed on parameters that define normal lactation.
Addressing diagnostics, Boss, Gardner, and Hartmann, provide strong rationale for several of the proposed indicators of normal function, including maternal plasma hormone levels and milk biochemistry across the progression of lactation. A key diagnostic that was not covered in their review is sufficiency of mammary glandular tissue. With a diagnosis of insufficient milk production, the “triple feeding” process for increasing milk output requires extraordinary commitment on the part of a new mother (every 2-3 hours, breastfeed, followed by breast pumping, followed by supplemental feeds of infant formula to the newborn). A diagnostic test for evaluating glandular tissue sufficiency would go a long way towards facilitating an individualized and sustainable breastfeeding management plan for affected mothers.
The use of reference values versus standards is the third example of the need for further consensus on defining normal lactation. The authors do rightly provide the WHO Child Growth Standards (4) as a frame of reference for defining the production of milk that supports “optimal growth and development.” Notably, the Child Growth Standards were explicitly designed to be just that—standards. The WHO team was very intentional in differentiating the 2003 standards on how children should grow from previous child growth reference values which simply depict what is occurring in a given place at a given time.
The authors rightly declare that the reference values provided in this review are a starting point of values available today. It is very exciting to look to the future as our community of lactation scientists hone in on the outcome measures, timing, and selection criteria, for sampling milk volume and composition across the stages of lactation in mothers across the globe with “normal lactation.” It took a 3-day conference with much belabored contributions by over 50 authors to produce Human Lactation: Milk Components and Methodologies, and surely consensus on globally relevant reference values for normal human lactation will likely take a similarly collaborative effort. ISRHML…this is your call to action!
References