Keywords
Diverticulitis, Risk factors, Prevention, Therapy, Diet, Microbiota, Colonic muscle, Drugs
Diverticulitis, Risk factors, Prevention, Therapy, Diet, Microbiota, Colonic muscle, Drugs
Colonic diverticula, sac-like herniations of the colonic mucosa and submucosa through the muscle layers, represent a common gastrointestinal condition in the Western world with a prevalence that grows with age, from less than 10% in people younger than 40 years of age to 50–66% after 80 years of age1,2. While most people with colonic diverticula remain asymptomatic, making diverticulosis not a disease per se, around 20% of patients will develop diverticular disease (DD) when they experience abdominal symptoms (i.e. symptomatic uncomplicated DD [SUDD]) and 1–4% will develop acute diverticulitis (AD)3. The natural history and pathophysiology of this clinical condition are still under definition. Clinical scenarios of DD and its natural history have been recently summarized4. In the past few decades, the increasing socioeconomic burden of DD has become evident, and efforts have been made to identify risk factors to establish relative preventive strategies and to achieve more standardized treatment approaches.
The aim of this review is to present updated evidence on AD epidemiology and relative open clinical questions and to analyze in detail predisposing and protective factors with the attempt to integrate their possible modes of action into the several pathogenic mechanisms that have been suggested to contribute to this multifactorial disease. Finally, evidence-based changes in therapeutic management will be summarized.
AD is an inflammatory process that involves one or more colonic diverticula, often associated with pericolonic inflammation that is classified as uncomplicated or complicated, the latter being characterized by the presence of abscesses, fistulas, peritonitis, and colonic stenosis. Complications most commonly occur with the first episode of AD5. AD clinical classification is mainly based on the use of modified Hinchey's criteria derived from contrast-enhanced computerized tomography (CT) imaging6 or on the more recent German guidelines7. However, an important role in AD diagnosis is also covered by abdominal ultrasound (US), which, in the hands of experienced investigators, can be used as a sensitive and specific diagnostic technique, limiting the use of CT after negative or inconclusive abdominal US7–10.
Recent epidemiological studies have confirmed the increase of hospital admissions for AD in recent years11. A previous epidemiological analysis, carried out in the USA in 201212, showed that diverticulitis was the third most-common gastrointestinal diagnosis from hospital admission and the leading indication for elective colon resection, with an increase of 41% from 2000 and an estimated cost of 2.6 billion dollars per year. A more recent analysis showed that the national cost of diverticulitis-related emergency department visits in the USA, from 2006 to 2013, increased by 105%13. Similarly, a recent observational analysis based on real-world data from an Italian region reported that direct healthcare costs for episodes of diverticulitis from 2008–2014 amounted to approximately 11.4 million euros, of which 95.5% was for hospitalizations14. With the growth of the population age, this significant economic burden is likely to continue to rise.
Currently, the increased rate of hospital admissions is especially evident among women and younger individuals5,15. Gender differences are age related in that hospital admissions for AD are predominant in men among subjects younger than 45 years of age, the opposite being true at older ages11. Besides, even if older patients display the highest rate of hospital admissions for AD, the increased rate of hospitalization is entirely accounted for by the younger cohorts of patients13.
Recent randomized controlled trials (RCTs) confirmed, and national guidelines recommended, that patients with uncomplicated AD are eligible for outpatient treatment without the use of systemic antibiotics, which should be used in complicated patients instead16–19. This conservative management strategy, if adopted, could positively influence the relative economic burden on health costs. However, the problem of AD recurrence remains a topic of debate.
In a recent retrospective population-based cohort study of 65,162 patients identified with a first episode of AD, the rate of hospital admission for recurrence was 11.2%20, lower than was previously reported21. This recurrence rate was greater in younger people and women5. Generally, the first episode is the most severe, with only 2% of recurrences resulting in a complicated case. Surgical treatment does not prevent the risk of recurrence, the rate of which is around 15%22,23.
Also, AD seems to predispose patients to developing long-term chronic nonspecific abdominal symptoms, similar to those observed in post-infectious irritable bowel syndrome24, with a higher prevalence after severe AD25. The proportion of patients that develop chronic abdominal pain after AD seems to be influenced by the type of treatment of the first episode, with a lower prevalence after elective laparoscopy (11%) compared to conservative treatment (39%)26.
As for now, the underlying mechanisms and risk factors that contribute to AD and its recurrence still need to be clarified. The actual therapeutic strategies aimed to modulate gut microbiota, such as rifaximin or probiotics, or to reduce mucosal inflammation, such as mesalazine, present a relatively poor efficacy for the prevention of the first AD episode (primary prevention) and its recurrence (secondary prevention) (see later). A definite assessment of AD-predisposing or -protective factors and these relative mechanisms could greatly contribute to patients receiving the best strategy for prevention, further reducing health costs, and improving the management of DD.
The identification of risk factors for AD has been the scope of several studies for the past few decades. In the last few years, the main concern has been to clarify whether or not factors associated with the first episode are also involved in AD recurrence27. Predisposing and protective factors for AD are summarized in Table 1. Among anthropometric features, obesity has been confirmed to be a risk factor for AD by a population-based cohort study28 and a recent systematic review and meta-analysis of prospective studies29, a risk previously reported in men in whom waist-to-hip ratio was also associated with the risk of diverticular complications30. As far as smoking is concerned, a lifestyle risk factor already known to be associated with AD, recent evidence showed that it further represents an increased risk of developing complicated AD31,32 and requiring surgery33. Red meat intake, particularly of unprocessed meat, was identified as the main dietary risk factor for diverticulitis in a prospective Health Professionals Follow-Up Study (1986–2012) of 46,461 men34.
A systematic review and meta-analysis35 confirmed that non-steroidal anti-inflammatory drugs (NSAIDs), which are already known to be risk factors for AD36 and perforation37, as well as steroids and opioids, presented an increased odds ratio for perforation and abscess formation.
Among protective factors, vigorous physical activity, as was previously reported38, was confirmed by a recent meta-analysis to be inversely related to diverticulitis29 but not by a population-based cohort study28. As far as diet is concerned, no recent further evidence is available on the lower risk of hospitalization for AD with a vegetarian diet and a high intake of dietary fibers39,40. Instead, statins, which have already been associated with a reduced risk of perforation35,37, have also been recently reported to be associated with a reduced risk of emergency surgery41. Finally, vitamin D serum levels, which when high pre-diagnostically have been associated with a low risk of AD among patients with diverticulosis42, have been related to the severity of DD endoscopic appearance43.
Some known risk factors still need to be confirmed. Evidence regarding alcohol consumption and DD are discordant, with some studies showing a positive association44,45 and others not46. Regarding drugs, the potential protective effect of calcium channel blockers in reducing the rate of diverticular complications observed in case-control analyses37,47 needs to be confirmed. Also, the recent epidemiological evidence that reports a higher prevalence of AD among women older than 50 years of age11,48, younger individuals5,11,13, and patients with end-stage renal disease49, as well as the association of cardiovascular disease and chronic obstructive pulmonary disease with complicated DD28,50, needs to be confirmed and explained.
Finally, recent studies corroborate the previous observation of a strong familial aggregation both in diverticulosis and in diverticular complications, suggesting a role for genetic factors51. A relationship has been reported between complicated diverticulitis and a single nuclear polymorphism in the TFNSF15 gene, a T-cell maturation receptor gene associated with other colonic inflammatory diseases52, and between early onset diverticulitis or unrelated sporadic diverticulitis and variants in the laminin β4 gene (LAMB4), which codes for constituents of the extracellular matrix that regulate the function of the enteric nervous system53.
Of note, even if clinicians frequently advised patients to avoid some foods, such as nuts, corn, and popcorn, these are not associated with an increased risk of diverticulitis54, and the same is true for fine or coarse grains28. Regarding the possible role of coffee, a previous study did not report any association between coffee use and DD44, similar to a recent cross-sectional study that did not observe any significant difference between coffee drinking and diverticulosis compared to SUDD or previous diverticulitis55.
An assessment of risk factors associated with recurrent AD is on its way, even if more studies are required to better target secondary prevention. Currently, increased risks of recurrent AD, obtained with a logistic regression model, have been shown to be younger age, female sex, smoking, obesity, a Charlson comorbidity index score of more than 20, dyslipidemia, and first complicated AD20. Other risks of recurrence are primary diverticulitis with abscess formation, an inflamed segment of more than 5 cm, multiple diverticula, and diverticula throughout the colon, with the risk of subsequent diverticulitis more than doubled after two earlier episodes of diverticulitis that further increases for every episode of recurrence56.
A unifying hypothesis to integrate the diverse risk factors and their contribution to the pathophysiology of AD has not yet been put forward. The current hypothesis asserts that the susceptibility of diverticula to inflammation is explained by local ischemia, retained stool, stercoral mucosal trauma by fecaliths, and diverticular wall distension that facilitates microperforations and favors bacterial translocation57. Inflammation and infections can spread transmurally (peridiverticulitis), ending in different types of AD complications. The improvement of the clinical outcome of the disease obtained with antibiotics supports an involvement of bacteria in most AD complications. A dual inflammatory–infective contribution might then be considered in AD pathogenesis58,59 with likely different interconnections if considering the luminal colonic and the extra-luminal microenvironments (Figure 1).
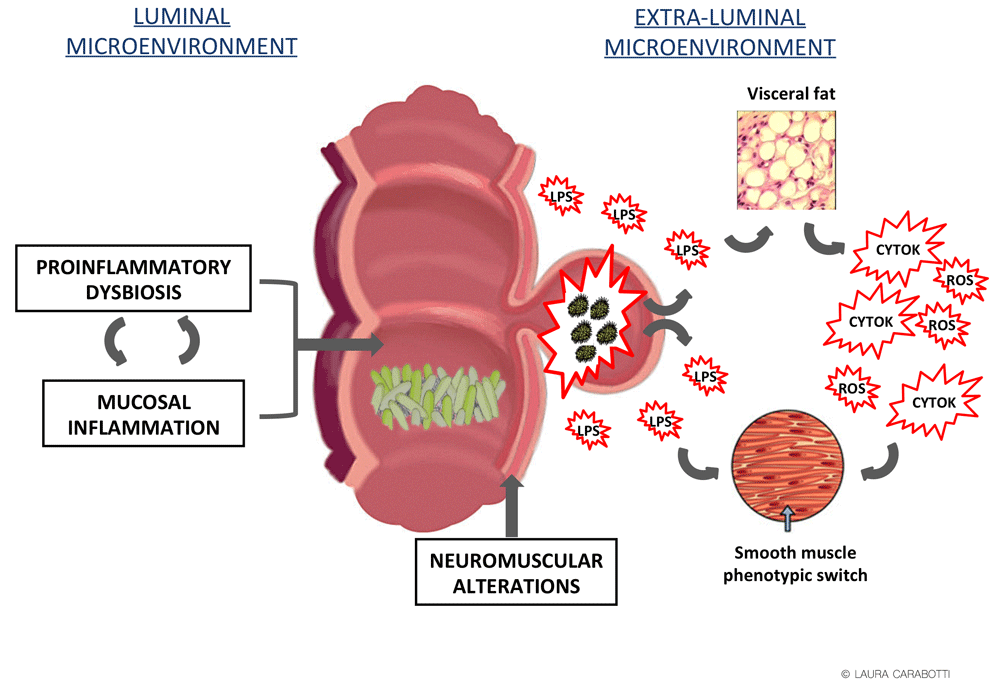
CYT, cytokines; LPS, lipopolysaccharide; ROS, reactive oxygen species.
The luminal microenvironment is dominated by microbiota that has been suggested to play an important role in the pathogenesis of the disease60. In uncomplicated DD, recent evidence indicates the presence of dysbiosis whose principal feature is the depletion of bacterial species with anti-inflammatory activity61 that likely favors mucosal inflammation. Recently, in rodent models, it has also been reported that aging-associated microbiota promotes inflammation and intestinal permeability and increases pro-inflammatory cytokines in the blood62, an aspect that could be relevant in DD, whose prevalence increases with age. Diet has an essential role in the maintenance of a healthy microbiota, and several types of food have been reported to be risk factors for AD likely through changes in microbiota composition. Among them, unprocessed red meat affects cecal microbial composition and metabolites63 and aggravates experimental-induced colitis, an effect counteracted by the co-consumption of resistant starch, which is considered a type of fiber that provides the benefits of both insoluble and soluble fibers64, an observation that could fit with the protective effects of a high-fiber intake in AD prevention. Fiber is known to possess anti-inflammatory effects and to have a eubiotic impact on gut microbiota65,66.
The presence of mucosal inflammation is still a matter of debate, except for DD-associated segmental colitis, a very rare mucosal inflammation sparing the rectum67. Evidence derived from studies carried out on small numbers of patients supports its presence. A strong positive correlation has been reported between activated CD68+/CD163+ macrophages and complicated sigmoid diverticulitis68, and an increase in the mast cell population has been found in the mucosal peridiverticular region61. iNOS and NO release, which are expressed in an inflammatory context, are increased in colonic mucosa, presenting a progressive trend from diverticulosis to SUDD with previous diverticulitis69. Similarly, higher chronic inflammatory infiltrates and inflammatory cytokine expression have been found in the affected tissue in patients after severe versus non-severe diverticulitis25. However, the presence of mucosal inflammation has not been confirmed by a recent study carried out in a large number (255) of patients, at least neither in diverticulosis nor in SUDD70. The absence of clear evidence regarding the efficacy of mesalazine in preventing both AD and its recurrence fits with these discrepancies.
The confirmation of an inflammatory mucosal context has several implications. Firstly, inflamed microenvironment in the gut drives bacterial dysbiosis71, favoring a vicious cycle between dysbiosis-driven inflammation and inflammation-driven dysbiosis. Secondly, mucosal inflammation and dysbiosis might lead to dysmotility72, another pathogenic factor associated with DD.
Dysmotility is present in both uncomplicated and complicated DD owing to a summation of different abnormalities73. Increased motility index and abnormal propulsive activity have been reported in patients with DD74, and enhanced contractile response has been observed in DD muscle strips, with upregulation of substance P and muscarinic M3 receptors75–77. In addition, specific abnormalities in longitudinal muscle relaxation and contents of neural nitric oxide and elastin have been reported78, confirming old observations of elastosis confined to the longitudinal muscle layer with subsequent shortening of the teniae coli79. A remodeling of the neuromuscular apparatus occurs in complicated DD, consisting of a muscular hypertrophy and architecture disarrangement with reduced content in density of myocytes and contractile myofilaments80, an enteric neuropathy with hypoganglionosis, an imbalance in neurotransmitters, a deficiency of glial neurotrophic factors coupled to a nerve fiber remodeling81–83, and an increase in collagen deposition in the colon wall84. An increase in collagen expression has been observed in human colonic circular and longitudinal smooth muscle cells from complicated DD, parallel to a switch from a contractile to a synthetic phenotype85. Of note is also that young patients with AD present an altered ratio between collagen subtypes with respect to female and older patients86.
As a whole, these alterations increase the rigidity and thickening of the colonic wall and subsequent loss of tensile strength that could squeeze the diverticulum orifice, favoring ischemia and stasis. The presence of a hypercontractile status may offer an explanation for the protective role reported for calcium channel blockers and conversely the increased risk of AD associated with opioids and NSAIDs. Opioids inhibit propulsive motility patterns87, while NSAIDs, by inhibition of COX enzymes, reduce prostaglandin-driven vasodilatation and muscle relaxation88. Of note, however, is that most of the available data have been obtained from surgical specimens of chronic stages of DD, and then it remains to be established whether neuromuscular alterations correspond to a primary or secondary post-inflammatory event. If the enteric neuromuscular pathology reflects a primary event, this could lead to disturbed intestinal motility patterns, increased intraluminal pressure, and ultimately to the formation of diverticula and relative inflammation, whereas if inflammatory events are considered as the driving force for the neuromuscular abnormalities, these changes would arise as secondary lesions resembling an associated pathology.
The scarce efficacy of the actual medical treatments aimed to counteract luminal pathogenetic factors, however, suggests that some other extra-luminal factors likely contribute to DD complex pathogenesis. Up to now, minor attention has been given to the alterations of the extra-luminal microenvironment. Microbiota-driven mucosal inflammation can favor the translocation of luminal bacteria from the diverticula wall to the perivisceral area with potential activation of receptors of the innate immune system, Toll-like receptors (TLRs), that can drive an inflammatory response in the surrounding tissues. In Crohn’s disease, the creeping fat surrounding the affected area was found to contribute to the overall inflammatory response89 and to be associated with a structuring/fistulizing course of disease90. Similarly, surrounding visceral fat has been hypothesized to play a pathogenic role in complicated DD91. In this context, the clinically significant link that has recently been reported between visceral fat and the severity of the presentation of diverticulitis is of interest, with visceral to subcutaneous ratio likely representing a predictive value of more complicated disease92, in accordance with obesity as a risk factor for AD.
Bacterial translocation from the diverticula wall to the perivisceral area can also activate TLRs expressed on human colonic muscular cells93. LPS binding to TLR4 is capable of triggering persistent and long-term oxidation-driven phenotypic myogenic cellular alterations94, which are conceivably associated with a smooth muscle cell ‘shift’ toward the synthetic phenotype95, resulting in a functional impairment of human colonic smooth muscle cells. Massive intramuscular fibrosis of both muscle layers has been described in DD96 and recently fibrosis, not predictable on endoscopic mucosal biopsies, has also been detected in DD submucosa97. Of note is that maximum colonic wall thickness is one of the factors used to predict recurrent diverticulitis98. This observed fibrotic trend could be influenced by NSAIDs, a known risk factor for AD and recurrences, that cause an enteropathy characterized by multiple short-segment strictures99. Part of the NSAID-induced damage in the gastrointestinal tract is caused by an uncoupling of mitochondrial oxidative phosphorylation that favors oxidative stress100. Likewise, the protective effect of statins could be related to their possible anti-fibrotic effects101. The involvement of peridiverticular tissues might then contribute to the decrease in compliance leading to stiffer tissue that is more susceptible to tears, especially under conditions of increased luminal pressures favored by muscular hypertrophy.
AD clinical classification and risk stratification is based on CT, which is able to offer a more comprehensive evaluation of uncomplicated and complicated forms, and severity and management are graded with the use of modified Hinchey's criteria6. More recently, a detailed classification of AD was proposed by German guidelines based on CT and clinical laboratory criteria7 (Table 2). Among AD, roughly two-thirds of patients present with uncomplicated diverticulitis102.
| Modified Hinchey Classification | German society Gastroenterology Classification | Management |
|---|---|---|
|
1a
Confined pericolonic phlegmon and associated inflammation without an organized fluid collection |
1a
No peridiverticulitis 1b Pericolonic phlegmon |
Consider outpatients management Not routine use of systemic antibiotic* |
|
1b
Pericolonic abscess less than 4 cm in size, adjacent to the area of diverticulitis |
2a
Concealed perforation abscess ≤1cm |
Hospitalization Bowel rest, parenteral fluids Systemic antibiotic |
|
2
Pelvic or inter-loop abscess, or abscess larger than 4 cm |
2b
Paracolic or mesocolic abscess >1cm |
Hospitalization Bowel rest, parenteral fluids Systemic antibiotic Consider Ultrasonography- or CT-guided drainage Consider surgery |
|
2c
Free perforation |
Hospitalization Bowel rest, parenteral fluids, Systemic antibiotic Consider laparoscopic lavage/surgery | |
|
3
Purulent peritonitis |
2c1
Purulent peritonitis | |
|
4
Fecal peritonitis |
2c2
Fecal peritonitis |
In this setting, an important role might be provided by abdominal US, which, in the hands of experienced physicians, can be used as a sensitive and specific diagnostic tool7–10. A multicenter study evaluating the accuracy of US compared with CT in unselected patients referred for acute abdominal pain to the emergency department showed that CT has a higher sensitivity compared to US in detecting AD (81% versus 61%; p=0.048)103. Currently, however, a strategy for providing CT after negative or inconclusive US has been proposed7,9,10.
There has been considerable focus over the past few decades on a conservative treatment strategy based on the administration of systemic antibiotics as well as on surgery either electively or in an emergency setting.
More than five years ago, Chabok et al. published the first prospective RCT in this area, the AVOD trial, showing that antibiotic treatment in patients with uncomplicated diverticulitis (without CT signs of abscess, fistula, or free air) neither accelerates recovery nor prevents complication or recurrences104. The most recent multicenter RCT of observational versus systemic antibiotic treatment (DIABOLO trial: amoxicillin plus clavulanic acid 1.2 g four times daily intravenously for at least 48 hours, after which the route was switched to oral administration of 625 mg three times daily) for a first episode of CT-proven uncomplicated AD (Hinchey stages 1a and 1b) showed that observational treatment without antibiotics did not prolong recovery and can be considered appropriate in patients with uncomplicated diverticulitis19. However, even if no significant differences between Hinchey stages 1a and 1b diverticulitis were found, it should be noted that the vast majority of patients included had a diagnosis of Hinchey stage 1a AD (90.1% in the observational and 94% in the antibiotic-treated group) with only a small percentage of patients with Hinchey stage 1b diverticulitis. Although these results suggested that antibiotics may not be necessary in patients with Hinchey stage 1b diverticulitis, currently no strong evidence to treat these patients without antibiotics is available, and more data need to be collected. In fact, a recent systematic review of national and international guidelines recommended treating small abscesses with antibiotics18. Moreover, only short-term results in omitting antibiotics were reported by both cited RCTs (AVOD and DIABOLO trials). More recently, the long-term effects of omitting antibiotics in uncomplicated AD were assessed after 24 months’ follow-up of the DIABOLO trial105. In the observational group, even if no significant differences were found in terms of recurrent diverticulitis and sigmoid resection, a higher number of elective resections was reported. Accordingly, the most recent European7–10,106,107 and American108 guidelines suggest the non-routine use of systemic antibiotics in patients with uncomplicated AD (Table 2). The need of hospitalization has been reconsidered as well, and a recent systematic review supported the safety, efficacy, and economic efficiency of an outpatient-based treatment approach109.
The management of complicated AD depends on its severity and complexity, requiring hospitalization, bowel rest, parenteral fluids, and, in selected cases, surgery. Antibiotic therapy is part of the management of complicated diverticulitis, and recent European guidelines7–10,106,107 are in accordance in recommending the use of broad-spectrum antibiotics (Table 2).
How to best treat complicated AD with surgery has been under debate and subject to notable changes recently. In particular, the number of episodes is not the only indication for surgery in patients with recurring diverticulitis: the individual case and course is also taken into account. A recent open-label randomized multicenter trial (DIRECT trial) randomized 109 patients after an episode of AD to receive surgical treatment or conservative management110. After a brief follow-up of 6 months, elective sigmoidectomy resulted in a better quality of life (assessed by many specific questionnaires) compared to conservative management. These results, even if innovative, might be affected by the heterogeneity of patients included (both patients with recurrent diverticulitis and patients with persistent abdominal complaints). In this setting, clinicians should carefully assess the relationship between symptoms and colonic diverticula differentiating abdominal complaints from irritable bowel syndrome, whose prevalence might increase after an episode of AD24.
Currently, the decision to perform an elective resection after one or more episodes of AD should be undertaken on a case-by-case basis, taking into account risk factors, complications, age, and severity of episodes as well as the patient’s personal circumstances and comorbidities (i.e. immunosuppressed patients)111,112.
Actually, the possible recognition of clinical or biochemical parameters that could be used to identify patients who progress from uncomplicated to complicated AD and to monitor the potential development of complications requiring immediate surgical intervention are matters of debate. Procalcitonin113 and neutrophil count and white cell to lymphocyte ratio114 have been proposed as accurate markers to differentiate complicated from uncomplicated diverticulitis, and higher levels of calprotectin, an inflammatory colonic mucosal marker, tend to be associated with more severe AD25. Clinical predictors of early recurrences up to 6 months after a first episode of AD appear to be high C-reactive protein (CRP) levels115, the presence of systemic inflammatory response syndrome, high pain score, and regular steroid or immunomodulator use116. For now, the proper timing of surgically treating AD remains undetermined, with surgical resection probably being reserved for patients with severe recurrences. Abscess formation should be treated by ultrasonography- or CT guided drainage while patients with signs of free perforation should undergo immediate surgery. Because of advancements in interventional technologies and laparoscopic treatment methods, surgical therapy is primarily aimed towards the control of emergency situations and avoidance of Hartmann’s procedures112.
Treatment protocols in DD, especially for AD prevention, have been recently summarized117. In AD prevention, epidemiological studies suggested that people consuming the highest quantity of fiber had a 41% lower risk of developing DD (0.59, 0.46 to 0.78; p<0.001) in comparison with those consuming less fiber46, with the reduced risk being strongest for cereal and fruit fiber39. This is in accordance with many national guidelines7–10,106,107 supporting their use.
Regarding rifaximin, recent evidence118 confirms that short monthly cycles of rifaximin with fiber supplementation may reduce the risk of AD occurrence, even if the number needed to treat (NNT) to prevent an episode of AD in one year was 57119. Despite the high NNT, Polish107 and Italian9,10 guidelines recommend the use of rifaximin associated with fiber intake. Data regarding the use of rifaximin for the secondary prevention of AD are weak, and recent guidelines did not agree with each other, with some for8–10 and others against7,108 its use. The most recent open RCT evaluated the efficacy of one-year intermittent rifaximin plus fiber to prevent AD recurrence120. After randomization, the underpowered number of patients included caused a study switch from evidence gathering to proof of concept. However, the authors reported that rifaximin was more effective compared to fiber alone in the secondary prevention of AD, with recurrence occurring in 10.4% versus 19.3% of patients, respectively (p=0.033).
Data regarding the use of mesalazine are inconclusive and more studies are needed, even if some positive results emerged for AD primary prevention121. Recently, the role of mesalazine in the secondary prevention of AD has been investigated by several RCTs122,123 that showed that mesalazine is not significantly superior to placebo in preventing AD recurrence. A recent Cochrane systematic review124 confirmed the uncertain role for mesalazine and its effects on AD recurrence, and a similar conclusion was drawn by a recent meta-analysis125.
In the last few years, the main goal that has been achieved in the management of AD is that uncomplicated AD can, to a larger extent, be managed on an outpatient basis with no or little supportive therapy, a strategy that will certainly impact on the health-related costs of this disease. A second achievement, one that needs more controlled studies, is the finding that one primary preventive intervention in AD is diet, specifically an adequate intake of fiber. The preferred type of fiber still needs to be elucidated considering that a high content in a FODMAP diet could cause an increase in colonic gas and fluids due to fermentation126.
Some other topics, mainly concerning AD recurrences, remain topics of debate. There is also a need of robust well-designed placebo-controlled RCTs that take into account the clinical history of the patient in order to achieve clearer evidence.
Because of an ascertained multifactorial pathogenesis of uncomplicated and complicated AD, it is likely that a single ‘causa prima’ will be not identifiable. In turn, it would be useful to stratify patients in order to separate those who could respond to lifestyle modifications from those with more aggressive disease who could be treated better with surgery. It is possible that besides age-dependent alterations, different pathogenic phenotypes might characterize more aggressive and complicated DD, such as those observed in younger people. A better stratification or a longer and more careful observation of the natural history of the diverticulosis versus diverticulitis process could be helpful to verify this hypothesis. It must be kept in mind that the actual therapeutic strategies (anti-inflammatory drugs, non-absorbable antibiotics, and probiotics), targeted towards luminal alterations, more easily demonstrated by the use of endoscopic samples, showed unsatisfactory efficacy in primary and secondary prevention. A better understanding of the different possible factors involved will be of great help in the planning of different possible therapeutic strategies, such as the use of nutraceuticals, and in pursuing tailored therapeutic algorithm strategies.
Carola Severi receives funding from the University Sapienza of Rome, 000324_17_RS_SEVERI_SAPIENZA_PROGETTI 2016.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 29 Jun 18 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)