Keywords
Gold mining, Migori, Nile tilapia, Oreochromis niloticus, total mercury
Gold mining, Migori, Nile tilapia, Oreochromis niloticus, total mercury
Mercury (Hg) is naturally present in the environment (air, soil, water) in trace amounts1. The three predominant forms of Hg are; elemental mercury (Hg0), ionic/inorganic mercury (Hg (II)/ Hg2+) and organic mercury (alkyl mercury compounds; where methylmercury; MeHg is the most important)1. Hg is considered by the United Nations to be one of the worst environmental pollutants in the World2. It is distributed in the environment by both natural and human activities including mining and smelting3, volcanic eruptions4, combustion of fossil fuels5, solid waste compost6 and geological weathering7. The burden of Hg in the atmosphere is reported to be increasing at a rate of 1.5% annually8,9. Contaminated soil and water are the principal conduits through which environmental Hg gains access to the food chain, notably in plants and livestock, including fish8,9. Once in the food chain, Hg can bioaccumulate, leading to adverse outcomes on human health and even death9.
All of humanity is exposed to some level of mercury1. However, the occurrence and severity of adverse health effects following exposure to Hg depend on various factors including the chemical form, dose, route and duration of exposure as well as the age/ developmental stage of the person exposed1. Exposure via diet may also be significant, particularly when foods such as fish and seafood are contaminated with mercury1. Populations at risk of mercury contamination include pregnant women, new mothers, fetuses of women exposed to methylmercury1, individuals with diseases of the liver, kidney, nervous system, and lungs1, neonates who consume contaminated breast milk10,11, recreational anglers, subsistence fish farmers, and people who are frequent consumers of large amounts of fish or meat from marine mammals (seals and whales)1. Individuals with high occupational exposure, those who use consumer products laced with mercury (skin lightening creams and soaps), partakers of traditional ethnic medicines containing mercury, and individuals that use dental amalgams are all at an increased risk of exposure1.
The nervous system is the primary reservoir for environmental mercury or its compounds and produces symptoms such as depression, amnesia, insomnia, tremors, and hearing loss9. Notwithstanding, the systemic distribution of mercury has the potential to produce a range of symptoms in different body systems including the cardiovascular, renal, digestive, hematological, pulmonary, immune, and endocrine systems9. Significant effects on these organ systems include cardiomyopathy, tubular necrosis, inflammatory bowel disease, aplastic anemia, bronchitis, leukemia, and painful/irregular menstruation9. Others include miscarriage, spontaneous abortion, stillbirth, low birth weight, neural tube deficits, craniofacial malformation, delayed growth, and cerebral palsy9.
Several countries and international organizations have established reference levels for daily or weekly methylmercury or mercury intakes based on risk assessments1. These levels provide estimates on the levels of mercury that are deemed to be safe. Reference intake levels for MeHg exposure range from 0.7-2 μg/Kg body weight per week1. Additionally, provisional tolerable weekly intakes (PTWIs) have been established by a joint FAO/WHO expert committee on food additives whose aim is to evaluate chemical contaminants in food1. This committee set the limit for total mercury at 5 μg/kg body weight and 1.6 μg/kg body weight for methylmercury1. According to the committee, the PTWI refers to the amount of a substance which can be consumed weekly over an entire lifetime without any significant risk to human health1. Moreover, the environmental protection agency of the United States has also developed a reference dose (RfDs) for mercuric chloride (0.3 μg/kg body weight/day) and methylmercury (0.1 μg/kg body weight/day)1. A reference concentration (RfC) for elemental mercury (0.3 μg/cubic meter) has also been developed by the same agency1. Exposures that are greater than the RfD imply that the potential for adverse health effects from mercury exposure also increases1. The Codex Alimentarius provides guideline levels of 0.5 mg for methylmercury per kg in non-predatory fish and 1 mg methylmercury/kg in predatory fish1. The United States Food and Drugs Agency has also set an action level of 1 mg methylmercury/kg in finfish and shellfish1. As for the European Union, a 0.5 mg mercury/kg has been set for fish, while Japan allows up to 0.4 mg total mercury/kg (or 0.3 mg methylmercury/kg) in fish1. Beyond these guidelines, some governments have also taken the initiative to provide dietary advice on the amount of fish to be consumed, the type of fish to be consumed and the groups of the human population that are at risk1.
Migori County is home to a vibrant small-scale artisanal gold mining community where mercury (Hg) is used to extract gold (Au). Up to 40% of the Hg used is lost to the environment during the process12. Various studies from the area aimed at monitoring the health of the ecosystem have painted a picture of contaminated soil, streams, and fish2,12,13. The government of Kenya has recently launched a program dubbed the Economic Stimulus Program (ESP), which aims to boost fish production in the country by promoting inland fish farming14. The program is heavily dependent on fast-growing species of fish such as the Nile tilapia15 and has been rolled out in about 160/290 administrative units in Kenya including many within the Migori gold mining belt. Moreover, some of the fish farms have been set up near active gold mining areas and the levels of T-Hg in water from the springs, underground streams and Lake Victoria that supply these fish farms are relatively unknown. Nile tilapia is also a popular and highly consumed source of protein for the local population. These conditions in unison necessitate the present study, whose general objective was to investigate the levels of T-Hg in pond water, sediment, and tissues of farmed and wild-caught Nile tilapia in Migori County. Specific objectives were: i) to investigate the T-Hg contamination in tissues (brain, liver, muscle) of farmed Nile tilapia fish from Nyatike and Rongo sub-counties (Migori County) as well as captured Nile tilapia from Lake Victoria, Kenya, ii) to determine the T-Hg contamination in pond water, and sediment collected from the Migori gold mining belt and how this contamination relates to contamination in the fish and iii) to evaluate the risk to human health following dietary exposure to T-Hg in different tissues of Nile tilapia. The information generated from this study may be necessary for fish consumers and policymakers, both locally and internationally.
Ethical approval was obtained from the Biosafety, Animal Care, and Use Committee of the Faculty of Veterinary Medicine, University of Nairobi (REF: FVM BAUEC/2018/148; Extended data, Figure S116). Guidelines on humane harvesting of fish17 were applied throughout the study. Extreme caution was exercised in handling concentrated mercury reagents and acids. Good laboratory practices (GLPs) were observed (use of gloves, gas masks, overalls, and fume chamber) at all times. Before the study began, it was ensured that the fume extraction system within the lab was in good working condition by following the laboratory’s standard operating procedures.
Purposive sampling was used to collect fish, sediment, and water samples across sites within the Migori gold mining belt. The number of samples collected are summarized in Table S1 (Extended data16). The levels of total mercury in the collected samples was evaluated in the lab by following the methods of the United States Environmental Protection Agency (US-EPA) and Analytical Methods for Atomic Absorption Spectroscopy by Perkin Elmer18–20 with slight modifications. (Modifications: 1. the target weight for all samples was 0.5 grams but for those samples whose weights were below this weight, the whole sample was weighed and processed. 2. The reaction tube was elongated by fixing a glass (Soselex) column on top to prevent bumping and spill over.)
The Migori gold mining belt covers five sub-counties namely Suna West, Nyatike, Rongo, Kuria West, and Kuria East within Migori County (Figure 1). Apart from mining, other economic activities undertaken in the region include livestock, maize, tobacco, and sugar cane farming. The main rivers that drain the area are the Mara, Kuja, and Migori2. Rongo and Nyatike sub-counties were selected for sampling. This is because, in addition to being within the gold mining belt, inland fish farming is widely practiced in the region. Therefore, ten sites namely Minyenya, Kamagambo North, Masara, Nyabisawa, Ndiwa, Kokaka, Luanda Nyira, Kamagambo South, Siginga beach and Sori beach located within the two sub-counties (Figure 2), and bearing coordinates between 0°6’11.16’’-0°52’51.6’’S and 34°7’8.4-34°37’55.2’’E were selected as study sites.
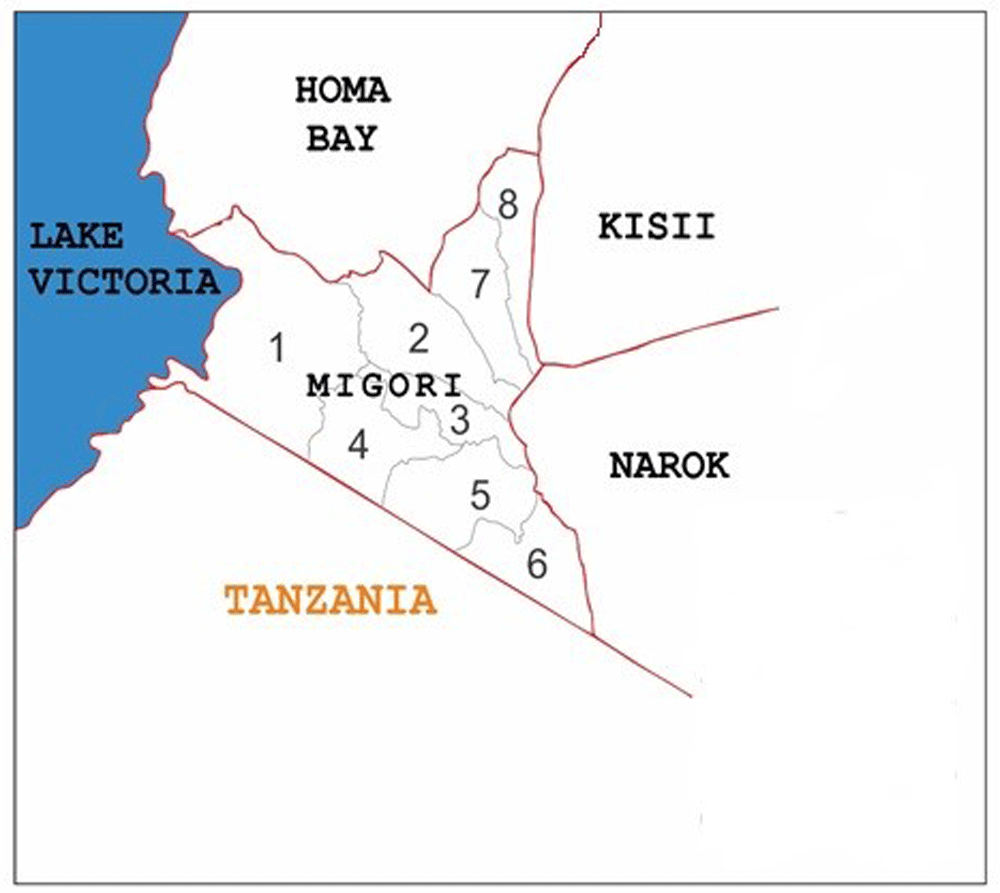
Key: 1: Nyatike, 2: Uriri, 3: Suna-East, 4: Suna-West, 5 Kuria-West, 6: Kuria-East, 7: Awendo, 8: Rongo.
By use of plastic trowels, near-surface (the top 5 cm) of fish pond sediments weighing approximately 1000 g was taken from each of the selected sites21, packed in plastic Biological Oxygen Demand (BOD) bottles (See details under extended data16. Table S2), labeled (location, sample type and the date of collection), kept on ice and transported to the laboratory where they were stored at -20° C until the time of analysis.
Trace metal clean procedures, as described by Shafer, Shelton, and colleagues22,23 were used to collect water samples. Briefly, water samples were collected in 250 ml metal-free plastic bottles and acidified to a pH <2 using 69% ultra-pure nitric acid (HNO3) (Extended data16, Table S2) to prevent adsorption of potentially harmful elements onto the interior walls of the storage bottles as well and minimize microbial activity. The resultant mixture was then filtered through a 0.45 µm pore filter (Extended data16, Table S2) and stored in 125 ml metal-free plastic sample bottles and refrigerated at -20° C until the time of analysis. Mercury in the filtrate (0.45 μm), also referred to as “dissolved” mercury was of particular interest in this study as this is what may have had measurable biological effects on aquatic organisms23.
Five tilapia fish (regardless of their sex) were captured from each site by using gill-nets (except Minyenya where four fish were sampled). Guidelines provided by the Faculty of Veterinary Medicine, University of Nairobi were adopted (REF: FVM BAUEC/2018/148; see Extended data16, Figure S1), and all efforts were taken to ameliorate harm to the animal model (fish). Additionally, guidelines on the humane harvesting of fish17 were adopted in euthanizing the collected fish.
A two-step process involving electro-narcosis and asphyxiation was used17. Fish were initially stunned in an electric field of 2.5V/cm at 1000 Hz to make them insensible to pain17. The absence of eye-roll reflex when the fish were moved from side to side was used as a confirmation that insensibility had been achieved17. Death was then induced by asphyxiation in the air for 10 seconds and was confirmed by the lack of movement of the operculum17. Stunning fish by use of low current of electricity is considered a humane method of euthanasia as this current makes the fish insensible to pain. The length of the fish was measured by laying an extended tape measure on a flat surface and placing the fish next to the tape measure. The total length was determined by measuring from the tip of the fish’s mouth to the tip of the caudal fin. The weight of each fish was measured on an analytical balance (Sartorius: SECURA® 324-101N). A blade (number 11) was used to make a slit incision about 3–5mm in length through the ventral abdominal wall and a 10 cm2 sample of muscle tissue was taken from each fish21. Liver and brain tissues were also harvested by carefully dissecting them out from the fish and transferring the tissues to aluminum foil by use of gloves. The collected samples were then transferred to plastic sample bottles which were labeled, and packed in self-zipping polyethylene bags, stored in ice in an ice cool box and transferred to a -20° C freezer awaiting further analysis. The characteristics of the ecosystem in fish ponds in the study area are summarized under Table S3 (Extended data16).
Pre-treatment of equipment and sample bottles. Precautionary steps, as described by Shafer and others23 were taken before using the equipment or sample collection bottles. Briefly, all equipment used for sample collection and storage of sediment, water, and fish samples were pre-cleaned using high-purity nitric acid and rinsed with sufficient quantities of reagent water. This was done to ensure that they were free of trace metals. After cleaning, the bottles were stored in double-bagged zip-lock polyethylene bags to ensure that no detectable metal contaminants were present in the sampling equipment.
Reagents. All chemicals and reagents used were of analytical grade (Merck, Germany; Sigma-Aldrich, France; Central Drug House, India; Fisher Scientific, UK). (Table S2, Extended data16). Double distilled, de-ionized water was used for preparing working solutions and for all analytical work. Standard stock solutions of mercury were prepared from a high purity standard stock solution with a concentration of 1000 parts per billion (ppb) and were diluted to the corresponding mercury working standard solutions (i.e.10 ppb, 20 ppb, and 30 ppb). These working solutions were freshly prepared daily by diluting an appropriate aliquot of the stock solution using 1M hydrochloric acid (HCl; Sigma-Aldrich) and diluting the resulting solution to 100 ml with reagent water.
Standard reference material for mercury in fish, i.e. the Community Bureau of Reference (BCR) – 463 (European Commission), was analyzed to ascertain the accuracy and precision of the experimental procedure. Alkaline solutions of sodium borohydride (NaBH4) were freshly prepared daily by dissolving 1.0 g of NaBH4 (Merck), and 0.25g of sodium hydroxide (NaOH) pellets (Merck) in 500 ml of distilled water. 3% v/v of HCl (Sigma-Aldrich) was used in the preparation of the carrier gas (Argon C-45). Stannous chloride (SnCl2) was freshly prepared by dissolving 62.5 g in 50 ml of 6 M HCl, the solution boiled for about 5 minutes, cooled, and nitrogen bubbled through it to expel any impurities of mercury. For sample digestion, 11 M nitric acid (HNO3; Merck), 18 M perchloric acid (HClO4; Merck) and HCl (Sigma-Aldrich) were used. Fused alumina anti-bumping granules (Merck) were used to avoid foam formation during sample digestion.
Equipment and apparatus. All glassware used in the analysis were soaked overnight in 10 % (v/v) nitric acid (HNO3), followed by washing with 10% (v/v) hydrochloric acid (HCl). They were then rinsed with double-distilled water and dried before use. Samples were weighed on an analytical balance (Sartorius: SECURA® analytical balance: SECURA 324-101N), and sample digestion was carried out in a steam bath (DK Heating Digester from Velp Scientifica) in the confines of a fume hood. A Varian Model Spectr AA 220Z atomic absorption spectrometer equipped with a mercury hollow cathode lamp was used for the analysis of the total mercury content of samples. Flow injection and cold vapor generation were done via a Varian model vapor generation accessory (VGA) 77. The analytical wavelength and slit widths were 253.7 nm and 0.5 nm, respectively. The Varian model, Spectr AA 220Z software (Version: Spectr AA), was used to monitor the output.
Sample preparation and digestion. All samples, certified reference materials, standards, reagent blanks, and spiked samples were processed using slightly modified methods of the United States Environmental Protection Agency (US-EPA) and Analytical Methods for Atomic Absorption Spectroscopy by Perkin Elmer18–20. Briefly, a top pan analytical balance (Sartorius: SECURA®analytical balance: SECURA 324-101N) was calibrated each morning before weighing samples. Samples were removed from the freezer and allowed to thaw for about an hour. A batch of samples (approximately 20 in number) was digested simultaneously. Then, a 0.3–0.5 g aliquot of a well-homogenized sample was weighed and placed at the bottom of a glass digestion tube. For samples that were less than 0.5 g (particularly the brain and liver tissues), the weighing was done so as to obtain as much of the tissue samples as possible to enable processing. Nine milliliters of concentrated nitric acid (HNO3), 3 ml perchloric acid (HClO4) and 1 ml of HCl (to stabilize the pH of the matrix) were slowly added to the glass digestion tube in a fume hood. The tube was allowed to stand at room temperature (in the fume hood) until the initial reaction subsided (about 15 minutes). A spoon scoop of fused alumina anti-bumping granules (Merck) was added to the solution to prevent it from bumping and spilling over. A glass (Soselex) column was fixed on top of the glass digestion tube to prevent spilling over in the event of frothing. The tube was then placed on top of a steam bath unit (DK Heating Digester; Velp Scientifica) which was programmed to heat gradually to 150 °C over 10 minutes. This heating was maintained for 120 minutes to complete dissolution. The tube was then removed from the steam bath, and the solution allowed to cool to room temperature over 30 minutes. The solution was then carefully transferred into a 100 ml flat-bottomed volumetric flask, the tube and column rinsed thoroughly with small amounts of distilled water and the resultant contents transferred into the flat-bottomed volumetric flask. Six milliliters of saturated potassium persulfate (K2S2O8) and 30 ml of potassium permanganate (KMnO4) was added to the solution and slightly shaken to mix. The resultant was left to stand for 40 minutes. Additional portions of the KMnO4 solution were gradually added until the resulting purple color persisted for at least 15 minutes. After thorough mixing, 6 mL of sodium chloride-hydroxylamine sulfate was added to reduce the excess permanganate (this was confirmed by the color change from purple to colorless). Reagent water was then added to the mixture up to the 100 ml mark and treated samples were then filtered through grade 541 (diameter 110 µm) filter paper. Five milliliters of stannous sulfate was then added to each of the treated samples. Thereafter, each sample bottle was immediately attached to the aeration apparatus (one at a time) of the cold vapor atomic absorption spectrophotometer.
Analytical quality control. Precautionary steps were taken to rule out any interference that may have arisen in the course of running the analysis. Briefly, method blanks were analyzed to ensure that all the materials (solvents, reagents, glassware, and other sample processing hardware) were free from artifacts or interferences which may have had the potential to compromise the integrity of the analysis. Interference from sulfide was abolished by the use of 5% w/v potassium permanganate (KMnO4). Excess hydroxylamine sulfate reagent (about 25 mL) was used to ensure that free chlorine was absent before the mercury was reduced and swept into the cell. Also, the dead air space in the BOD bottle was purged before adding stannous sulfate.
A preliminary run without reagents (using reagent water) was also used to rule out interference by volatile organic materials. Moreover, the accuracy of the procedure was determined by analyzing three certified reference materials (CRMs) namely; Tuna fish muscle fapas CRM, BCR 463 from Community Bureau of Reference, European Commission, vegetable puree CRM (EU) and fish CRM (EU). Recovery studies were performed by adding a known amount of standard solution of mercury chloride to some samples (spiked samples), which were then taken through the digestion procedure. The concentration of mercury in the resulting solutions was then analyzed.
Analysis of mercury in collected samples. The optimum operating temperature of the CVAAS instrument was set at 18 °C, and the circulating pump was adjusted to continuously pump at the rate of 1 L/min. Maximum absorbance was noted within 30 seconds. The bypass valve was then opened, and aeration continued until absorbance returned to the minimum value. The bypass valve was then closed, the fritted tubing removed from the BOD bottle, and aeration continued. The measurement time was set at 5 seconds, with a pre-reading delay of 45 seconds in between readings. Aliquots of 1.0, 2.0 and 3.0 mL of the mercury working standard (containing 0.1 mg/L or 1000 ppb) of mercury and 1 ml of fuming (37%) hydrochloric acid (HCl) solution was transferred to a series of 100 mL volumetric flasks and made up to the mark with reagent water. The standards had 10, 20, and 30 ppb of mercury, respectively. A calibration curve was automatically generated from the instrument’s software, plotting the absorbance of the standard against the concentration of mercury in parts per billion. The absorbance of the samples and standards were determined from the recording device and corresponding mercury concentrations tabulated.
Evaluation of the degree of sediment contamination. The quantitative geochemical accumulation index (IGeo) was used to evaluate the level of mercury contamination in fish pond sediments collected from different sampling sites. This method applies the formula proposed by Müller24 to calculate the degree to which sediment is contaminated by mercury. Thus, IGeo= log2 (Cn/1.5×Bn), where; IGeo= the geochemical accumulation index, Cn=sediment metal concentration and Bn=geochemical background value of the metal. In this study, the global mercury background value was taken as 0.05, as described by Reimann25. Accordingly, mercury pollution in collected sediments was classified into seven categories (0–6) as: class 0 (unpolluted; IGeo≤0), class 1 (unpolluted to moderately polluted; 0≤IGeo≤1), class 2 (moderately polluted; 1≤IGeo≤2), class 3 (moderately to strongly polluted; 2≤IGeo≤3), class 4 (strongly polluted; 3≤IGeo≤4), class 5 (strongly to extremely polluted; 4≤IGeo≤5), and class 6 (extremely contaminated; IGeo>5)24.
Risk-based consumption limits. Guidelines set by the United States Environmental Protection Agency (US-EPA)26,27 were used to calculate the potential health risk from consumption of Nile Tilapia sampled in the region. An assumption was made that the ingestion dose was equal to the absorbed dose of Hg, as has been described previously by Chien and colleagues28. Calculations on mercury consumption limits were based on the US-EPA reference dose (RfDo). The ratio between exposure and the reference dose indicated by the target hazard quotient (THQ), were calculated on the standard assumption of an integrated US-EPA risk analysis model. The methods described by Copat et al.29,30 were used to estimate the daily intake per meal (EDIm) and the target hazard quotient (THQ) as below;
Where EDIm is the estimated daily intake of mercury per meal size; MS is the standard weight portion of fish (230 g) for adults according to Hosseini et al.31; C refers to the concentration of mercury in mg/kg wet weight according to Marrugo-Negrete and colleagues32; BW is the body weight (assumed to be 70 kg for an adult)29. According to the US-EPA, the RfDo for T-Hg is 0.1 μg/g/day26.
For non-carcinogenic effects, the maximum allowable fish consumption rate in meals/week (CRmw) according to the US-EPA26 that would not be expected to cause any chronic systemic effects were calculated as below;
Where; MS is the standard weight portion of fish (230 g) for adults according to Hosseini et al.31, and C refers to the concentration of mercury in mg/kg wet weight according to Marrugo-Negrete and colleagues32. Considering an average adult body weight of 70 kg, the Hg USEPA Acceptable Daily Intake (ADI) can be approximated as 7 µg/day/adult (49 µg Hg/week)26,31.
Data for mercury analysis was expressed as the mean ± standard deviation. One-Way Analysis of Variance was used to analyze the levels of mercury in fish tissues across the sites. Tukey’s HSD test was used as a post-hoc test. Pearson’s correlation coefficient was used to determine whether there were any relationships between mercury levels in water and those in fish tissues, levels of mercury in sediment and those in fish tissues, and the levels of mercury in fish tissues and the pH of the pond water. The same test was also used to determine relationships between the levels of mercury in fish tissues and pond water pH, temperature, weight, and age of the fish. The t-test was used to analyze the relationship between the level of mercury and the type of fish culture practiced. Microsoft Excel (2016) and Statistical Package for the Social Sciences (SPSS, version 20.0) were used for statistical analysis. p≤0.05 was considered significant in all cases.
The mean levels of T-Hg were highest in the fish brain tissues and ranged from 0.128±0.021 µg/g wet weight (n= 5, 95% CI) at Nyabisawa in Nyatike sub-county to 3.128±1.421 µg/g wet weight (n= 4, 95% CI) at Minyenya in Rongo sub-county (Table 1 and Underlying data).
The fish muscle tissues were the second most contaminated with mean T-Hg levels ranging from 0.179±0.020 µg/g wet weight (n= 5, 95% CI) at Kamagambo South in Rongo sub-county to 0.917±0.099 µg/g wet weight (n= 5, 95% CI) in Minyenya. Table 1. The fish liver tissues were the least contaminated tissues with mean T-Hg levels ranging from 0.103±0.118 µg/g wet weight (n= 5, 95% CI) in Kamagambo South, Rongo sub-county to 0.588±0.374 µg/g wet weight (n= 5, 95% CI) in Kokaka, Rongo sub-county (Table 1).
There was a significant difference (p=0.00) in the mean levels of T-Hg in fish brain tissues sampled from the different sites (Table S4, Extended data16). Further evaluation of this variation revealed that the mean levels of T-Hg in fish brain tissues was significantly different between Nyabisawa and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036), Kamagambo South and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036), Sori beach and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036), Siginga beach and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036) and between Masara pond and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036), Sori beach and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036), and Kamagambo North and (Luanda Nyira, Kokaka, Ndiwa and Minyenya) (p=0.036) (Table S5, Extended data16).
There was a significant difference (p=0.00) in the mean levels of T-Hg in fish muscle tissues sampled from the different sites (Table S6, Extended data16). Further evaluation of this variation revealed that the mean levels of T-Hg in fish muscle tissues was significantly different between Kamagambo South and Minyenya (p=0.043), Kamagambo North and Minyenya (p=0.043), Kamagambo North and Masara pond (p=0.022), Kamagambo North and Siginga beach (p=0.022), Luanda Nyira and Masara pond, and Luanda Nyira and Siginga beach (p=0.022). (Table S7, Extended data16).
There was a significant difference (p=0.00) in the mean levels of T-Hg in fish liver tissues sampled from the different sites (Table S8, Extended data16). Post hoc analysis of this variation established that the mean levels of T-Hg in fish liver tissues was significantly different between Kamagambo South and (Sori beach, and Minyenya) (p=0.005), Masara pond and (Sori beach and Minyenya) (p=0.005), and between Ndiwa and (Sori beach and Minyenya) (p=0.005) (Table S9, Extended data16). The mean levels of T-Hg in all fish tissues (brain, liver, muscle) collected from the different study sites were found to be significantly higher than the critical value of 0.2 µg/g wet weight (n=49, 95%CI) which is the consumption limit for at-risk human populations (Table 3). However, only the fish brain tissues were found to have mean levels of T-Hg that were significantly greater than the critical value of 0.5 µg/g wet weight (n=49, 95%CI) which is the critical value for the general population (Table S10, Extended data16).
The levels of T-Hg in sediments ranged from 0.208±0.000 to 1.113±0.008 µg/g wet weight (n= 3, 95%CI) with six of the eight sample sites being moderately polluted (1≤IGeo≤2), whereas two sites (Minyenya and Kokaka; Rongo sub-county) were strongly polluted with total mercury (3≤IGeo≤4; Table 2).
An increase in the mean levels of T-Hg in the fish pond soil sediments coincided with an increase in the mean levels of T-Hg content in fish brain tissues (r= 0.528, sig. = 0.001, 95%CI) as well as an increase in the mean levels of T-Hg in fish muscle tissues (r= 0.524, sig. =0.001, 95%CI; Table S11, Extended data16). However, there was no significant correlation between the mean T-Hg levels in pond sediments and tilapia liver tissues (sig. =0.923, 95%CI; Table S11, Extended data16).
The mean levels of T-Hg in the water samples collected from the different sites ranged from 0.002±0.000 µg/ml to 0.004±0.001 µg/ml (n=3, 95% CI). (Table 3)
| Site | Level of total mercury (mg/L) |
|---|---|
| Minyenya Kamagambo North Masara Nyabisawa Ndiwa Kokaka Luanda Nyira Kamagambo South | 0.002 0.004 0.003 0.004 0.004 0.002 0.004 0.004 |
The water samples from all the sites were found to have mean T-Hg levels that were significantly greater than the critical value of 0.0001µg/ml (n=8, sig. = 0.000 at 95%CI) set by the food and agriculture organization for unpolluted surface waters33. (Table S12; Extended data16). An increase in the mean levels of T-Hg in fish pond water coincided with a decrease in the mean level of T-Hg in fish brain (r= - 0.402, sig. = 0.011, 95%CI) and muscle (r= - 0.616, sig. =0.000, 95%CI) tissues. (Table S13) Extended data. There was no significant correlation between the mean levels of T-Hg in pond water and the mean levels of T-Hg in fish liver tissues (sig. =0.874, 95%CI; Table S13, Extended data16).
An increase in the pH of fish pond water coincided with an increase in the mean levels of total mercury in fish brain tissues (r=0.48, p<0.05). (Table S14) Extended data. An increase in the temperature of the water in fish ponds coincided with an increase in the mean levels of total mercury in fish brain tissues (r=0.404, p<0.05; Table S14, Extended data16). The larger the fish, the lower the level of total mercury in fish brain tissues (r = -0.623; p<0.05; Table S14, Extended data16 and Underlying data34). There was no relationship between the age of fish and the level of total mercury in the fish brain, liver, and muscle tissues. (Table S14, Extended data16).
The type of fish culture practiced did not affect the mean levels of total mercury in fish brain tissues (Table S15, Extended data16). However, the type of culture practiced affected the mean levels of total mercury in fish muscle and liver tissues (Table S15, Extended data16).
The health risk indices from feeding on fish sampled from the different study sites are summarized in Table 3. Based on these results, feeding on fish sampled from Sori beach, Masara, Siginga beach, and Kokaka was associated with significant exposure to mercury as the CRmw was the least (Table 4). On the other hand, the risk of exposure to mercury was least in Kamagambo South on account of the large consumption rate in meals per week (Table 4).
Fish can take up mercury from their environment and store it in relatively high concentrations in their tissues35. The levels of mercury recorded in fish tissues in this study were generally high when compared with the joint WHO/FAO critical reference guideline values for the general population36. Also, the levels of mercury, particularly in fish brain tissues were generally high when compared to the WHO/FAO critical reference values for at-risk populations (pregnant women, and children under five years)37. Our findings contradict those of Campbell and colleagues21 who reported that the levels of mercury in several species of fish captured from African Lakes, including Lake Victoria had mercury levels that were within WHO limits.
In this study, the levels of total mercury in fish tissues were in the order; brain>liver>muscle. This suggests that brain tissues of Nile tilapia in the region may have been releasing mercury at a slower rate than the liver and muscle tissues. It may also indicate that the distribution and accumulation of mercury in tissues of Nile tilapia seems to be biased towards the brain tissue. The role of metabolism on mercury bioaccumulation in the different tissues cannot be ruled out either. It could be that the metabolic rate in the brain tissues of Nile tilapia within the region was higher than other tissues and this may have predisposed fish brain tissues to a higher assimilation efficiency for methyl mercury than other tissues. Methyl mercury has a strong affinity for sulfhydryl groups in tissues and accumulates to a higher concentration in brain, muscle, and kidney tissues38.
From the study’s findings, the fish brain may be the most dangerous part of the fish to eat as methyl mercury (a significant contributor of total mercury) is not eliminated from fish tissues by any practical cooking method38. This is worrying since the local community in Migori associate feeding on the fish brain with an increase in the intelligence quotient of the consumer.
The World Health Organization has developed guidelines on the maximum mercury intake per week, which serves as an advisory to fish consumers and in effect, help to avoid the accumulation of mercury in the body39. Our findings on risk-based consumption limits were specific for each tissue (brain, liver, and muscle). In reality, however, humans usually consume the whole fish. Therefore, considering the cumulative mercury concentration in the fish tissues (brain+liver+muscle), our results of the EDIm, THQ, and CRmw suggest that Kamagambo South and Nyabisawa were the areas where fish can be consumed with low risk to human health. Conversely, Masara, Kokaka, Siginga beach, and Sori beach are the areas where the risk to human health from consuming fish is highest.
Higher levels of mercury in soil sediments coincided with higher levels of mercury in fish tissues (notably brain and muscle). According to Gupta and colleagues40, sediments are the most important reservoir of metals and other pollutants in the aquatic environment. The fact that heavy metal contamination in sediment has been shown to affect bioaccumulation of metals in aquatic organisms41 may partially explain why brain and muscle tissues in our study had high levels of mercury. However, it is unclear why there was no association between the levels of mercury in soil sediments and the levels of mercury in fish liver tissues. More studies may be needed to explore this phenomenon further.
The observation of low levels of total mercury in water sampled from fishponds may have something to do with the pH of the water, the presence of suspended solids in the ponds, as well as adsorption and precipitation processes. These factors have previously been shown to have the potential to remove metals such as mercury from solutions in the form of sulfides under anoxic conditions42. Also, inorganic mercury and methyl mercury (components of total mercury) have been reported to form complexes with naturally occurring dissolved organic carbon, thereby reducing the amount of mercury available in water43. This study revealed that as the content of mercury in water increased, the quantity of mercury in fish tissues (brain and muscle) decreased. According to past research, most of the mercury that accumulates in higher trophic level species originates from consumed food rather than direct aqueous accumulation44,45. However, this does not explain why there was a decrease in the quantity of total mercury in fish tissues (brain and muscle) as the level of mercury increased in the water. This observation warrants further research.
This study established that as the pH of pond water increased, so did the level of mercury in fish tissues (brain but not liver and muscle). Previous reports indicate that once mercury is assimilated by fish, it is distributed via the blood and stored in various tissues46. Thus, in the process of excretion, there is a transfer of mercury between ‘donor’ and ‘receiver’ organs, thereby implying that fish tissues are bound to have varying concentrations of mercury. Based on this, we speculate that in our case, alterations in the pH of fishpond water within the Migori gold mining belt appear to have favored bioaccumulation and distribution of mercury to the brain tissue as a receiver organ relative to liver and muscle tissues. Also, we posit that alterations in pH may also have influenced the release of mercury from brain tissues of Nile tilapia relative to liver and muscle tissues. The lack of a relationship between pH and mercury levels in liver and muscle tissues of Nile tilapia seems to suggest that the rate of bioaccumulation, distribution, and release from these tissues may not be dependent on changes in the pH of water in fishponds within the Migori gold mining belt. This study’s findings suggest that high water temperature was associated with higher levels of total mercury in fish brain tissues (but not liver and muscle tissues). Mechanistically, rising temperature in water may lead to an increase in the feeding rates of fish in response to higher metabolic demand47. Such an increase in food consumption could result in higher methyl mercury uptake and accumulation47. This may be due to a temperature dependent distribution and release of mercury from tissues of Nile tilapia in the study area. The findings suggest that the larger the fish, the lower the levels of mercury in fish brain tissues. Controversy abounds over the relationship between the weight of fish and the levels of mercury in fish tissues. Some studies have reported positive correlations between the two variables48 while others have reported negative correlations49. These differences may be due to differences in species, growth rates, body condition, and other bio energetic-related factors.
This study’s findings seem to suggest that the type of culture practiced in the Migori gold mining belt has some effect on the bioaccumulation and distribution of mercury in tissues of the Nile tilapia (particularly in liver and muscle tissues). The polyculture system (use of multi-species of fish) appears to be associated with high levels of mercury in fish tissues. It may be that polyculture practiced in fishponds within the Migori gold mining belt may expose Nile tilapia to unique physiological stressors that increase the rate of metabolism in fish tissues. Consequently, the rate of consumption of food among these fish rises resulting in a more pronounced uptake of mercury from food as well as a diminished elimination from the tissues. There is a need for studies to further investigate these findings.
Interpretation of this study’s results may be limited by several factors. First, the statistical approach adopted may have ignored spatial correlations between sampling points and thus may have missed relevant information. Secondly, the fish diet has been reported by other studies to be one of the major pathways for the overall accumulation of mercury. In this study, the levels of mercury in feed and how potentially this would have translated to bioaccumulation in fish tissues were not investigated. Furthermore, this study used a single species of fish as a bio-indicator of pollution. Multi-species’ comparisons covering different feeding habitats of fish and a wide range of age categories may provide data that may facilitate stakeholders to distinguish recent exposure from the long-term load. Finally, this study did not explore the seasonal variation of mercury concentrations and what effect this might have had on bioaccumulation of mercury in the tilapia fish.
Levels of mercury in tissues of Nile tilapia in the Migori gold mining belt are above-recommended limits. This study’s findings suggest that there is a significant health risk associated with mercury exposure through the consumption of Nile tilapia within the Migori gold mining belt. Thus, there is a need for fish consumption advisories about mercury in the Migori gold mining belt.
This project contains the following underlying data:
Figshare: Underlying data (1) of the study 'Levels of Mercury in Nile tilapia (Oreochromis niloticus), water, and sediment in the Migori gold mining belt, Migori, Kenya, and the potential ramifications to human health by Kola et al. https://doi.org/10.6084/m9.figshare.8869967.v350
This project contains the following underlying data:
Raw data on the total mercury levels in water, sediment and tissues of Nile tilapia in the Migori gold mining belt.xlsx (Spreadsheet of recorded mercury levels)
Figshare: Underlying data (2) on the study 'Levels of Mercury in Nile tilapia (Oreochromis niloticus), water, and sediment in the Migori gold mining belt, Kenya and the potential ramifications to human health by Kola et al. https://doi.org/10.6084/m9.figshare.8869964.v234
This project contains the following underlying data:
Figshare: Extended data on the study 'Levels of Mercury in Nile tilapia (Oreochromis niloticus), water, and sediment in the gold mining belt of Migori, Kenya, and the potential ramifications to human health' by Kola et al. https://doi.org/10.6084/m9.figshare.8870053.v416
This project contains the following extended data:
Kola-supplementary material.docx (word document containing supporting materials)
○ The ethical approval form of the biosafety, animal use and ethics committee of the University of Nairobi. (Figure S1)
○ The total number of sediment, water, and fish tissues collected from the ten sites in the study area. (Table S1).
○ A summary of the reagents and chemicals used in the analysis. (Table S2).
○ Characteristics of the ecosystems of the fish ponds where water, sediment, and fish were collected. (Table S3).
○ Overview of the computed data (statistical analysis). Table S4 to Table S15.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This study was funded by the Partnerships for Enhanced Engagement in Research (PEER) Science project and the United States Aid for International Development [PV 43358/34 awarded to SK]. The funds facilitated transport across the sites as well as collection and analysis of data.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to acknowledge the staff of the Migori County Fisheries Department notably Ms. Ruth, Mr. Joseph Oruru and Mr. George for their assistance in the identification of sites, and facilitating coordination with the fish farmers as well as sample collection. Special gratitude is extended to technical staff at the Department of Public Health, Pharmacology and Toxicology, University of Nairobi notably Mr. Joseph Nderitu, Mr. Nduhiu Gitahi, Mr. Maloba and Mr. Kimotho for facilitating the storage and processing of the collected fish samples. Also, we appreciate the invaluable help and guidance offered by staff at the analytical chemistry laboratory of the Kenya Plant Health Inspectorate (KEPHIS) who contributed to the successful analysis of the collected fish samples. Lastly, we would like to thank Dr. Gerald Muchemi, Dr. Florence Mutua and Mr. Billy Odhiambo for their contribution to data analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Aquatic ecology, mercury pollution.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Food chemistry, toxicology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Jul 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Regards.
Regards.