Keywords
BCGitis, Mycobacterium bovis, Bladder cancer, sepsis
BCGitis, Mycobacterium bovis, Bladder cancer, sepsis
Most bladder carcinoma are located superficially, described as a non-muscle invasive bladder cancer, which allows a therapeutic approach with local immunotherapy1. The Bacillus Calmette Guérin (BCG) vaccine was initially produced as a vaccine against tuberculosis. BCG is an attenuated live strain of Mycobacterium bovis and has been widely used as an immunotherapy over the last few years2. Treatment with the BCG vaccine is well tolerated by over 95% of patients3.
The most frequent side effects of immunotherapy with the BCG vaccine are limited to the bladder – bacterial or chemical cystitis and haematuria. Although rare, systemic side effects can be mild or severe and they range from fever and influenza-like symptoms to BCG-induced lung infection, liver toxicity and BCG sepsis. Sepsis was estimated to affect 0.3% of patients treated with BCG instillation3. When a disseminated infection is present it can be described as BCGitis, with the lung and liver being the most frequently affected organs (50% and 61%, respectively)4
A diagnosis of BCGitis can be validated by direct staining (Ziehl-Neelsen or auramine-rhodamine), histological observation (presence of a granulomatous pattern with or without caseous necrosis) or the results of polymerase-chain reaction (PCR) assays. However, the rentability of all options is low, leading to increasing difficultly in establishing the diagnosis4,5.
We hereby report a rare case of septic BCGitis.
A 61-year-old Caucasian male with a medical history of pulmonary embolism and bladder cancer (pTaG1), who had been treated with the BCG vaccine for the last 15-months (a total of 15 instillations) was admitted with a 3-week history of fever (38°C), asthenia, anorexia and a weight loss of 6kg.
A week prior to admission (virgula) the patient presented with dysuria and pollakiuria, justifying an empirical treatment for a urinary tract infection with ciprofloxacin (500mg bid) but without response. the urine culture requested came back negative.
The patient’s general state was aggravated with persistent fever, tachycardia and increased inflammatory markers (CRP 46mg; normal range, <5mg/L), elevated liver enzymes [AST (46U/l); ALT (64U/I)], leading to hospital admission with a diagnosis of sepsis (Day 1; Table 1). An empirical broad-spectrum course of antibiotics with ceftriaxone (2gr) and gentamycin (500mg) was instituted for 5 days. A septic screen was performed, which grew no microorganisms.
Haemoglobin (Hb) is expressed in grams per liter; Neutrophil cells, platelets, lymphocytes and monocytes are expressed as elements /mm³; Aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (gGT), and alkaline phosphatase (ALP) are all expressed in international units per liter; Total bilirubin is expressed in milligrams per decilitre and C-reactive protein is expressed in milligrams per litre.
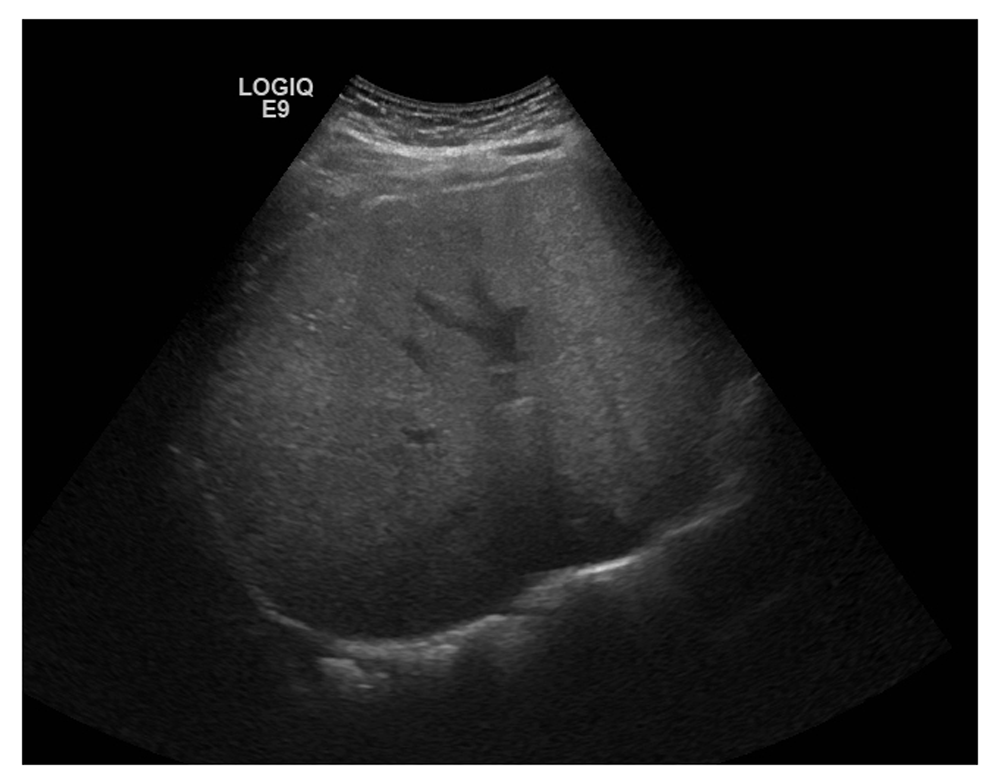
After 3 days of hospital stay, the patient’s status continued to deteriorate with persistent fever, hypotension and an increase in CRP to 76mg/L (Day 3; Table 1). No abnormal lymph nodes were observed. An abdominal ultrasound revealed a hyperechoic liver lesion with preservation of length and liver contour (Figure 1). This evidence (virgula) combined with the knowledge that there had been a previous recent administration of immunotherapy with BCG 3 weeks before the onset of the presented clinical symptoms, lead physicians to perform a liver biopsy.
A liver biopsy was conducted revealing multiple small epithelial granulomas without caseous necrosis compatible with granulomatous hepatitis. Ziehl-Neelsen staining and Löwenstein–Jensen medium culture were negative. A PCR assay for M. tuberculosis complex (MTB) was also negative both in the liver biopsy and urine sample.
Due to continuous clinical worsening of the patient’s condition until day 7 and aggravated sepsis with persistent fever, hypotension and increased CRP to 186mg/dL under broad-spectrum antibiotics, an empirical regimen with isoniazid 300mg, rifabutin 300mg and ethambutol 1200mg was started for 3 months. Given its interaction with rivaroxaban, rifampicin was switched to rifabutin. In a matter of days, the clinical outcome with resolution of the fever and normalized the inflammation markers at day seven from admission (Table 1). Moreover, within one week of therapy, CRP, AST and ALT revealed a notorious decrease, with a complete normalization within 11 days upon treatment institution.
The patient´s therapeutic regimen included isoniazid 300mg, rifabutin 300mg and ethambutol 1200mg for the first 3 months and the subsequent 3 months with isoniazid and rifabutin with good adherence and physiological tolerance. After 2 years of treatment, the patient remains in remission.
Our case highlights the rare but dangerous systemic secondary side effects of BCG instillations that characterize (singular) BCGitis. Evolution to a multisystemic disease deserves attention because its diagnosis can be a challenge to any physician – unspecified symptoms could also be present in acute infection, autoimmune disorders, evolution of malignancy or drug toxicity.
The pathophysiology of BCGitis is not yet well understood. Previous studies have suggested that it is the result from hypersensitivity, with a diffuse granulomatous reaction without isolation of M. bovis, while others consider it a disseminated disease of M. bovis4.
The identification of Mycobacterium requires a combination of tests because there is a poor reliability of PCR for MTB, Ziehl-Neelsen or auramine-rhodamine stains and also with Löwenstein–Jensen medium culture4,6. The histological finding of granulomatous lesions remains the main support to the diagnosis when it is not possible to identify Mycobacteria, as we describe in this case. However, the steatotic liver pattern presented in Figure 1 supports the presence of liver inflammation and the need of a liver biopsy.
In sensitive strains, the use of empirical antibiotherapy with fluoroquinolone could decrease the accuracy of Ziehl-Neelsen stain, Löwenstein–Jensen medium culture and PCR for MTB.
Options for treatment regimens and duration of therapy also remain under discussion; however, treatment options that have been accepted vary from 6–9 months4,5. Our patient was treated for 6 months, which proved to be adequate for treatment of BCGitis. The interaction of rivaroxaban with rifampicin forced a switch to rifabutin.
Corticosteroids should only be used in severe cases, due to the lack of evidence in mild and moderate presentations4.
Finally, the present case confirms the good clinical and laboratorial response upon institution of empirical therapy, with complete recovery. Moreover, two years after BCGitis, the patient is stable but remains under surveillance.
We describe in detail a very rare case of sepsis secondary to immunotherapy with BCG for a non-muscle invasive bladder cancer. Most frequently, patients have local manifestations, however systemic infection may also occur. The diagnosis can be challenging as other infections and malignancies must be first ruled out. Nonetheless, physicians ought to be familiar with bcgitis and upon diagnosis, prompt therapy must be started.
Written informed consent for the publication of this case report and any associated images was obtained from the patient.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Desmet M, Moubax K, Haerens M: Multiple organ failure and granulomatous hepatitis following intravesical bacillus Calmette-Guérin instillation.Acta Clin Belg. 67 (5): 367-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Immunology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dermatology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Aug 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)