Keywords
Diamond-Blackfan anemia, adipose tissue-derived mesenchymal stem cell, intravenous injection
Diamond-Blackfan anemia, adipose tissue-derived mesenchymal stem cell, intravenous injection
Diamond-Blackfan anemia (DBA) is a rare congenital bone marrow disorder, which characterized by erythroblastopenia1. The symptoms of DBA are generally presented in infancy, with broad congenital abnormality including growth retardation, defect of heart and urogenital system, malformation of hands, and cleft lip/palate2. It has been known that a mutation in the genes coding ribosomal proteins (RP) is responsible for DBA2. The large proportion of DBA patients have mutations detected in RP genes of the small (RPS7, RPS10, RPS15A, RPS17, RPS19, RPS24, RPS26, RPS27, RPS28, RPS29) or the large (RPL5, RPL11, RPL15, RPL18, RPL26, RPL27, RPL31, RPL35, RPL35A) ribosomal subunit3. In rare cases of DBA, two pathogenic X-linked mutations (GATA1, TSR2) were reported recently2. These ribosomal abnormalities in DBA are known to lead to the impairment of erythropoiesis4.
The treatment of DBA has conventionally focused on the mitigation of anemia resulting from impaired erythropoiesis. The administration of corticosteroids and blood transfusions have been commonly adopted for DBA patients2. However, steroids have various adverse effects after long-term use and the life-long transfusions for the patients who do not respond to steroids can also lead to problems, such as iron accumulation3. Allogenic hematopoietic stem cell (HSC) transplantation is considered a more fundamental approach for the steroid-refractory patients and to avoid iron accumulation from long-term transfusion5. Currently, the bone marrow from HLA-matching donors or cord blood transplantation have been carried out, and the positive outcomes have been reported6. However, allogenic HSC transplantation has considerable difficulties, such as the recruiting of HLA-matching donors, the in vitro propagation of HSCs, and the various complications from immunosuppression therapy, graft versus host disease (GVHD) etc.3.
Mesenchymal stem cells (MSCs) have been noted for a novel alternative in cell therapy in various diseases over past decades7,8. MSCs have been known for the effective tissue repair, angiogenesis, and the stimulation/mobilization of endogenous stem cells9–11. Adipose tissue-derived MSCs (AdMSCs) have shown several advantages over MSCs from other origins in safety and effectiveness, including a less invasive sampling procedure, abundant cell populations from tissue harvested and high capacity of proliferation12,13. Here we assessed the potential efficacies and the safety of intravenous administration of autologous AdMSCs in patients with DBA for the first time.
A girl aged 11 years and 10 months was admitted to our hospital (Bethesda Hospital, Yangsan, Korea). She presented with severe anemia, left blepharoptosis, and polydactylism in the right thumb at birth, and was diagnosed with pure red cell aplasia and congenital dyserythropoietic anemia in infancy. After seven transfusions in the first year of life, prednisolone or methylprednisolone were orally administrated at 1 mg/kg/day for 4 years from the age of 2.5 years. The patient was finally diagnosed with DBA due to a mutation in RPS24 at the age of 8 years by genetic analysis. The immediate family history was not identified. At that time of the visiting of the hospital for the treatment of AdMSC, the patient had already undergone four surgeries on her left blepharoptosis and had mild ptosis of the right eye; polydactylism in right hands was corrected after the surgery. The administration of autologous AdMSCs for DBA was approved by the Korean Ministry of Food and Drug Safety with Investigational New Drug Application for Emergency Use (Approval No. 20180101335). Written informed consent was acquired from the patient and patient’s parents before the initiation of treatment. This study was conducted in compliance with the Helsinki declaration and approved by Institutional Review Board of Bethesda Hospital (Approval No. 2018-FEB01).
The isolation and characterization of the autologous AdMSCs were performed using the previously established culture protocol14 under good manufacturing practice (GMP) conditions in the Stem Cell Research Institute of R Bio (Seoul, Republic of Korea). Briefly, the abdominal subcutaneous adipose tissue was obtained through liposuction, and digested with collagenase I (Gibco/Life Technologies, Grand Island, NY, USA). After centrifugation, the pellet was resuspended in DMEM (Invitrogen, Carlsbad, CA, USA)-based media containing 0.2 mM ascorbic acid and 10% fetal bovine serum (FBS; JR Scientific, Woodland, CA, USA). The cell fraction was cultured overnight at 37°C/5% CO2, and the cell adhesion was checked after 24 h. The cells were maintained for 4 to 5 days until confluent (passage 0). When the cells reached 90% confluency, they were subculture expanded in keratinocyte SFM-based media (Invitrogen, USA) containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/ml rEGF, and 5% FBS until passage 3. Before transporting the cells for administration, aliquots of the AdMSCs are tested for cell viability, fungal, bacterial, endotoxin, and mycoplasma contamination and immunophenotype for MSCs. Cell viability evaluated by trypan blue exclusion was >91%, and no evidence of bacterial, fungal and mycoplasmal contamination was observed. The AdMSCs showed a homogenous population of cells with high positive marker expression levels of CD73 and CD90 at a high level of >85% and >99%, respectively. Negative markers of CD31, CD34, and CD45 were expressed at a very low level of <0.6%. AdMSCs finally adjusted to 1 × 108 cells in 100 ml of saline were injected intravenously for 1 hour. The intravenous administrations of AdMSC were carried out five times in 2-week intervals (June 21–Aug. 14, 2018), and the patient was instructed to visit the hospital to check the various assessments for safety (vital signs, physical examination, laboratory tests, adverse events, and serious adverse events).
The treatment schedule is showed in Figure 1. After 3 and 6 months from the first administration of AdMSCs, red blood cell (RBC) count, the hemoglobin value, hematocrit, reticulocyte percentage were assessed for the efficacy (Table 1).
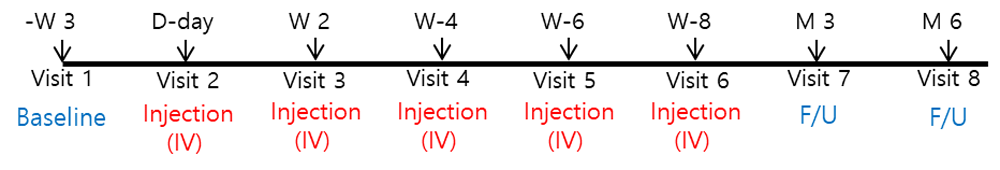
IV, intravenous; F/U, Follow-up; M, months; W, weeks.
| Pretreatment | Post-treatment | Reference Interval | |||
|---|---|---|---|---|---|
| Baseline | Month 3 | Month 6 | Month 9 | ||
| Height (cm) | 151.4 | 154.4 | 155.3 | 151.7* | |
| Weight (kg) | 50.0 | 50.9 | 50.2 | 43.7* | |
| RBC (×106/μl) | 1.58 | 1.81 | 2.15 | 2.38 | 5.7-8.8 |
| Hemoglobin (g/dl) | 5.6 | 6.4 | 7.5 | 8.3 | 12.0-18.0 |
| Hematocrit (%) | 16.9 | 19.2 | 22.9 | 26.1 | 37.1-57.0 |
| WBC (×103/μl) | 5.1 | 6.6 | 5.7 | 5.9 | 6.0-19.5 |
| Reticulocyte (%) | 1.3 | 1.6 | 1.9 | 0.5-2.5 | |
There were no side effects or adverse events observed during the administration of the autologous AdMSC. Most criteria of the assessment for the vital signs, physical examination, and laboratory tests showed the values within normal or clinically meaningless subnormal range. There was also no concomitant medication during the treatment of AdMSC. Although showing subnormal values, the number of RBC, hemoglobin level, and hematocrit level were improved after the systemic administration of AdMSC. The patient showed steady improvement in anemia after the treatment of AdMSCs; the RBC count was 2.38 ×106/μl hemoglobin level was 8.3 g/dl and hematocrit level was 26.1% after 9 months from the first administration of AdMSC (Table 1).
DBA is a congenital hematopoietic disorder caused by the mutation in genes of the ribosomal protein1. The mutation of RPS19 is founded in the approximate 25% of DBA cases, and RPS24 in 2% of cases1,4,15. The defects of RPS19, PRS24, and other genes involved in ribosomal biogenesis result in the impairment of the cell cycle and the protein synthesis rather than the differentiation process of the erythropoiesis, and finally give rise to the depletion of the erythrogenic progenitors16,17. Recently, gene therapy has been recommended as an emerging treatment, which could remedy the shortcomings of conventional treatment and be the fundamental cure3. However, the collection of genetically reprogrammed cells (induced pluripotent stem cells, HSCs, adult fibroblast, etc.) shows very low efficiency, and the risk of mutation limits the collecting of the clinical data and the application of treatment of humans3,4. AdMSCs, with its feasibility of the application, has already been identified as having potential for the treatment of various diseases, from chronic degenerative conditions to congenital defects or orphan diseases14,18. Autologous MSCs from patients with genetically defective disease could alleviate the patients’ own condition. The previous studies demonstrated that the autologous MSC from the patients with sickle cell anemia19 and autosomal dominant polycystic kidney disease20 were functionally compatible, and could be effective in the patients’ own status. The mechanisms of these efficacies of MSC are known to largely depend on the paracrine activity, which secretes the extracellular vesicles (exosomes) containing various cytokines, miRNAs, growth factors, and endocrine factors involving the bone regeneration, angiogenesis, immunomodulation, cellular proliferation, differentiation, recruiting of the endogenous stem cells21,22. Although the parameters were still in the subnormal range in the present case, the patient with DBA, who presented with severe anemia, showed a stable improvement from the anemic state after the intravenous administration of AdMSCs, without any other medications. The erythroid cells from patients with DBA have been reported to show defective expansion and proliferative properties23. However, the DBA erythroid colony formation could be enhanced under the presence of the exogenous stem cell factor (SCF, a KIT ligand) in vitro23–25. SCF is a major hematopoietic factor; the stromal cells from patients with DBA normally express SCF mRNA transcripts26. SCF secreted from the arterial endothelial cell in the bone marrow is essential for the intrinsic HSC27. AdMSCs have superb angiogenetic properties, which are considered to secrete the exosomes containing SCF, colony stimulating factor, interleukins and the other hematopoietic factors18,27,28. Further, AdMSCs stimulate the regeneration of endothelial cells through the paracrine angiogenetic factors, such as vascular endothelial growth factor, basic fibroblast growth factor, etc.22. In this regard, the encouraging outcomes in the present case can be assumed that there would be the direct hematopoietic supports from the patient’s own AdMSCs, and/or indirect hematopoietic help from the endothelial cells induced by the patient’ own AdMSCs. However, due to the restrictive case number, the lack of assessment of the extent of angiogenesis and the hematopoietic analysis after treatment with AdMSCs, there is a limitation to evaluate the potential of AdMSCs for erythropoiesis. Nonetheless, the present case report dealt on the first patient’ own AdMSCs administration for DBA treatment and gave the possibility that the intravenous administration of autologous AdMSCs can be a safe alternative for DBA patients.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of the patients’ clinical details was obtained from the parents of the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular basis of inherited and congenital bone marrow failure syndromes
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 13 Sep 19 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)