Keywords
Cardiovascular disease, Tajikistan, Hypertension, Public Health
Cardiovascular disease, Tajikistan, Hypertension, Public Health
Non-communicable diseases (NCDs) are the leading cause of death worldwide, causing 41 million deaths in 20161. Half were due to cardiovascular diseases (CVD)2. Primary health care (PHC) systems are an essential component required to tackle this global burden3. While strong PHC systems based on the principles of family medicine contribute to achieving universal health coverage, better health outcomes and economic and social development, many nations lack PHC capacity4,5. To help national governments develop primary health care capacity for NCDs, the World Health Assembly endorsed the WHO Global Action Plan for the Prevention and Control of NCDs 2013–20206,7.
A country of the former Soviet Union, Tajikistan is located in Central Asia; the capital city is Dushanbe. Ranking among the poorest in the WHO European Region, Tajikistan had a gross domestic product (GDP) of 796 USD per capita in 20168. The WHO Regional Office for Europe continues to support Tajikistan to implement the Global Action Plan, including support for the development, testing, and national scale-up of evidence-based clinical guidance and health policy for the prevention and management of NCDs.
NCDs are a major burden in Tajikistan. The age-standardized NCD mortality rate was 685.3 per 100,000 in 20159. In 2016, the probability of dying prematurely (between the ages of 30 and 70 years) from CVD, cancer, diabetes, or chronic respiratory disease was 25.3%; like most populations, this rate is higher for men than women10.
The Ministry of Health of Tajikistan and the World Health Organization Regional Office for Europe recently completed a population STEPS survey (unpublished by WHO) which indicated that 25.7% of men are current users of tobacco (including smokeless tobacco) although rates are low amongst women (0.2%)11. For cultural reasons, alcohol use is relatively low with only 9.4% of men and 0.2% of women identified as current drinkers, and lifetime abstention is very high. The prevalence of obesity is 11.9% in women and 15.4% in men, and around half (46.7%) the adult population are overweight.
The prevalence of raised blood pressure (BP) (defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg or currently taking medication for raised blood pressure) among the adult population is 32.2%. Less than half of men (44.3%) have never had their blood pressure measured; the same is true for 24.7% of women. Nearly all (96.9%) adults have never had cholesterol measured. One-in-seven (13.8%) people aged 40–69 years have a 10-year fatal or non-fatal CVD risk of over 30% (including those with an existing CVD).
The health system of Tajikistan is still shaped by the country's Soviet legacy and the pace of reform has been slow12. The country has the one of the lowest total health expenditure (THE) per capita in the WHO European Region, estimated to be 6.88% of GDP in 2014, and the proportion of THE that is financed privately through out-of-pocket payments (OOP) is 61.69%; second highest in the WHO European Region8. Although formally free, OOP expenditure for PHC is common and expenditure on pharmaceuticals is the biggest financial burden.
Recent reforms have aimed to strengthen PHC, but success has been mixed. In 2010, Tajikistan launched the National Health Strategy for the period 2010–202013, which recognized the importance of health system strengthening, and highlighted the development of PHC based on family medicine practice as a top priority. The National Programme on the Development of Family Medicine 2011–2015 in Tajikistan had the goal of ensuring the sustainable development of PHC according to the principles of family medicine. Some substantial improvements were achieved, including trainings for the health workforce, the review of clinical protocols, promotion of quality of care, increasing capacity in family medicine, improvements in evidence-based practice, and greater availability of resources. Nevertheless, challenges have been highlighted, including insufficient pace and scale of initiatives, and the scope of work and distribution of family doctors across the country14. Although some computers have been installed in district health care facilities to introduce electronic submission of statistical reports, the vast majority of rural health clinics rely on paper records which need to be completed manually, hindering reliable data collection, processing and analysis12.
In Tajikistan, health services are delivered at five levels: rural (village), district (rayon), city, oblast (regional), and republican (national). In villages, PHC services are provided in rural health centres (RHCs) with a family medicine doctor or health houses with feldshers, family medicine nurses and midwives.
In order to build capacity in PHC and ultimately prevent premature mortality from major NCDs, from 2013 to 2015 Tajikistan endeavoured to adapt and pilot the World Health Organization Package of Essential NCD Intervention for Primary Healthcare in Low Resource Settings (WHO PEN)5. WHO PEN includes simplified clinical protocols which together cover the integrated management of hypertension and diabetes, as well as education and counselling on healthy behaviours aimed to prevent CVD. The central strategy of this integrated approach is the use of total cardiovascular risk assessment to stratify and target individuals at high CVD risk, a process considered to be a “best buy” intervention by the WHO15.
These early efforts led to limited success. A national PEN steering group was established in 2012, facilities were assessed for access to essential medicines and technologies, national clinical guidelines were updated to include the WHO/ISH risk prediction chart16, and family doctors and nurses from 9 pilot facilities received two-day training on the WHO PEN protocols during 2013/14. Apart from a “refresh” training for selected clinics two years later, there was no further intervention, and the lack of systematic monitoring and evaluation made it difficult to demonstrate any significant change in clinical practice, disease detection, disease control rates or clinical outcomes.
In 2016, Tajikistan agreed to embark on a pilot of the global WHO HEARTS initiative supported by WHO17. Work on this was able to proceed in earnest in 2018 with the publication of the HEARTS technical package which aimed to provide a strategic approach to improving cardiovascular health in countries18. It comprises six modules and an implementation guide to support Ministries of Health to strengthen CVD management in primary health care settings.
Led by the Ministry of Health and Social Protection, the WHO PEN steering group was expanded to include chief specialists, district and rural health facility managers and clinicians, the Republican Clinical and Training Center of Family Medicine and the Service of State Supervision for Medical Activities and Social Protection of the Population of the Republic of Tajikistan. The national steering group is supported by an international team of experts coordinated jointly by the WHO Regional Office for Europe and the WHO Country Office in Tajikistan. Tajikistan has a particular interest in systems monitoring, and in developing sustainable and scalable approaches, building upon other initiatives in PHC reforms and quality improvement.
The aim is to determine the feasibility of implementing and evaluating essential interventions for the management of hypertension and prevention of CVD in PHC in Tajikistan.
In order to achieve this aim, the objectives are to:
1. Determine the baseline performance of PHC services with respect to essential interventions for the management of hypertension and prevention of CVD;
2. Assess the ability to conduct practical trainings for primary care doctors and nurses and implement Tajikistan-adapted HEARTS tools, in one pilot region of Tajikistan;
3. Estimate the change in clinical practice with respect to essential interventions for the management of hypertension and prevention of CVD after 12 months of implementation;
4. Determine the feasibility of collecting quantitative data required for future studies of effectiveness from routine clinical data.
As part of a larger programme of implementation research, we have previously developed an approach to evaluating and piloting essential interventions for NCDs in low-resource settings, and have adapted this methodology to suit the context of Tajikistan19.
The general approach to developing, piloting, and evaluating essential interventions for the management of hypertension and prevention of CVD in PHC in Tajikistan are outlined in the following seven steps. A more detailed methodological description is presented in the section that follows.
Step One: Refresh clinical decision-making tools. Tajikistan national guidelines for PHC exist in a printed book format, and these books are available and used by family doctors. The national guidelines for hypertension (updated in 2018) endorse the use of WHO/ISH CVD risk charts, and some family doctors were previously trained on the use of WHO PEN. However, there is a lack of simple clinical decision support tools, such as laminated one-page algorithm cards for the treatment of hypertension. As such, the following tools will be adapted to be in-line with existing national guidelines, used for training, and disseminated to PHC workers in intervention clinics.
HEARTS Hypertension Protocol – Diuretic as First-line Treatment
HEARTS WHO PEN Diabetes Protocol
WHO PEN Protocol 1 – adapted to integrate with HEARTS Hypertension Protocol
WHO PEN Protocol 2 – adapted to integrated with HEARTS Hypertension Protocol
Body-Mass Index Calculation Table
WHO CVD Risk Charts – WHO/ISH no cholesterol charts
Integrated Management of Hypertension and Diabetes Algorithm (HEARTS Risk Module)
AUDIT and Fagerstrom Clinical Decision Support Tools
While the content of these tools is consistent with national guidelines, a ministerial order may be required to inform clinicians in the intervention clinics that it is appropriate to follow these updated versions of the already approved national guidelines.
Step Two: Update training package for primary health care workers. There is an existing two-day training developed for the implementation of WHO PEN in Tajikistan. This training package will be reviewed for overall content, pre- and post-training evaluation of participant knowledge, adult learning methods, practical exercises, consistency with refreshed clinical decision support tools and protocols, and assessment of practical clinical skills.
Furthermore, based on the previous experience of implementing WHO PEN in Tajikistan, the following topics have come to light as important to emphasize in the updated training package:
Organization of care
Task sharing between doctors and nurses
Meaningful clinical record keeping
Approach to managing a patient without laboratory cholesterol tests
Appropriate and timely follow-up of patients
Optimization of nurse home-visit tasks to include greater focus on underserved populations at risk for CVD (e.g. older men)
Measuring inequalities in care and outcomes by gender
Raising population awareness of cardiovascular diseases
Step Three: Collection of baseline data. Baseline data will be collected from all clinics in the included district before implementation (i.e. training) begins. Data for quantitative indicators will be extracted by randomly sampling individual paper-based patient records from all PHC units using a standardized data collection instrument. This will be done by a small team of specially-trained national experts. After analysis, each health clinic will receive a summary report of their facility’s performance with respect to key project indicators. This report will not be powered for statistical significance, but will be used as a quality improvement exercise to show how routine clinical data can be used to improve clinical practice, for example by setting improvement targets.
Step Four: Training staff in intervention clinics. All doctors and nurses from the PHC centres in the intervention arm will be invited to be trained together by a national team of experts in groups of approximately 30. It is estimated that up to 100 health workers will be trained in total. Given the human resources, the trainers will be themselves a group of family doctors and nurses, rather than narrow medical specialists or academics, so as to maintain direct relevancy to practical aspects of PHC services.
The training will be two-days; therefore it is estimated that two trainings can be conducted in one week (Monday/Tuesday and Thursday/Friday). Thus, 60 participants can be trained per week; two weeks will be required to train all doctors and nurses in the pilot district. A ministerial order will be required to relieve the health staff from their duties to attend the training.
Step Five: Implementation of protocols and implementation coaching. Trained participants from intervention clinics will then be free to implement the clinical protocols and change their clinical practice, without incentives, for 12 months. During this time, a team of national and district experts will be created to offer support (distance and on-the-job) to the PHC centres. These teams will visit each clinic once every three months (for a total of four visits) and use a set of standardized instruments to review performance and provide constructive and timely feedback to the health facility manager and individual clinicians. The tools used during the support visits and implementation coaching will include:
HEARTS Treatment Supervision Form – with minor adaptations to local context
HEARTS Patient Interview Report Card – with minor adaptations to local context
HEARTS Summary of Supervision Visits – with minor adaptations to local context
Structured Interview and Debrief Conversation Template – to be developed
In addition to clinic visits every three months, representatives from each clinic will be brought together after 6 months of implementation for a workshop. This will be an opportunity for the project management team to provide support based on the needs identified in clinic visits, to encourage target setting, quality improvement and peer support, as well as gather more information about implementation barriers.
Step Six: Collection of follow-up data. After 12 months, using the same method and data collection instruments used to collect baseline quantitative data (Step Three), data will again be extracted from randomly selected individual paper-based patient records from both intervention and control health centres. As was done at baseline, each health clinic will receive a summary report of their facility’s performance with respect to key project indicators comparing baseline to follow-up.
One-on-one semi structured interviews will be conducted with doctors, nurses, and health facility managers in the intervention clinics at 12 months follow-up, and these qualitative data will be analysed thematically for explanatory themes.
Step Seven: Evaluation of results and sharing experience. The findings of the quantitative and qualitative analyses will be integrated in a final report and shared with key stakeholders, including health staff from the participating primary health care centres. The results will also be shared at a national conference and in an open-access peer reviewed journal, in order to inform the future development of primary health care capacity of Tajikistan and other similar settings.
A pragmatic, sequential mixed methods explanatory design, composed of quantitative and qualitative strands will be used (Figure 1). The quantitative strand will be weighted more than the qualitative strand. A mixed methods design was chosen because it allows for the use of qualitative data to enlighten and explain the quantitative findings, but due to limited capacity to conduct qualitative research in Tajikistan, the qualitative strand will be weighted less than the quantitative strand.
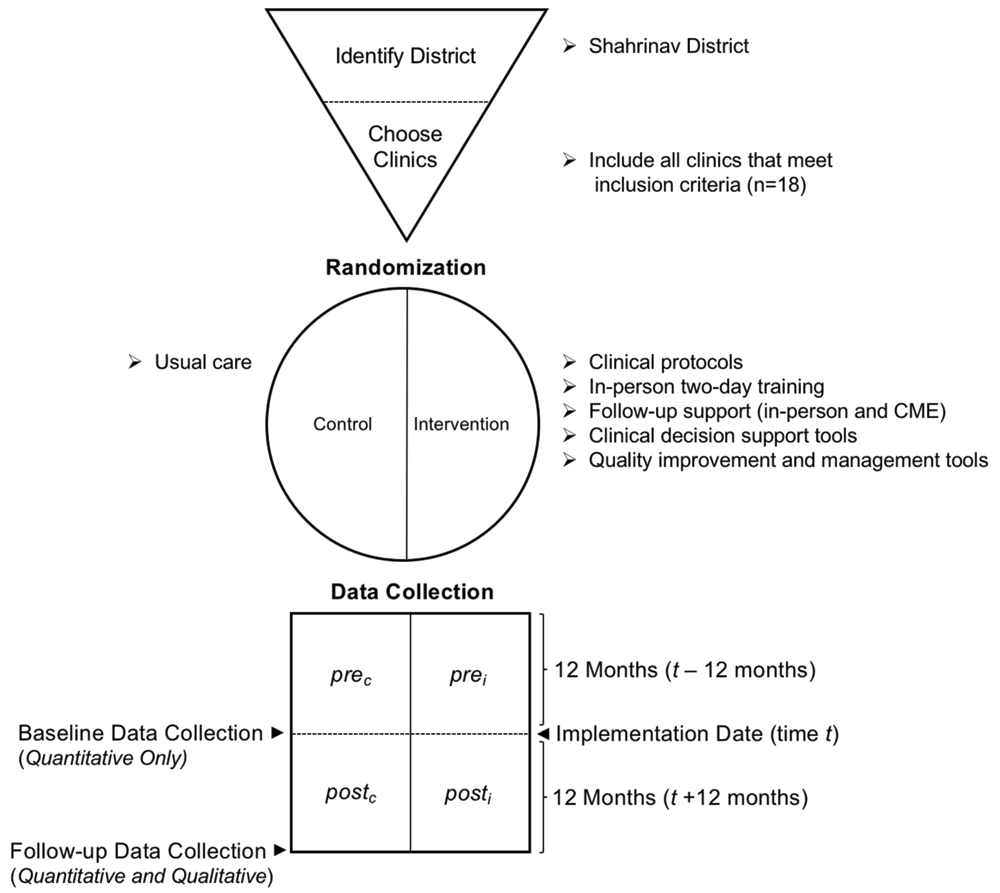
Due to resource constraints, and several ongoing PHC reform projects, a single district was nominated by the Ministry of Health and chosen for implementation (rather than multiple districts). All PHC in the district that meet inclusion criteria will be included; half will be randomly assigned to the intervention arm and half to the control arm. Baseline data will be collected from all clinics in the intervention arm and the control arm using the same standardized data extraction form.
The national working group initially nominated four districts for consideration which had participated in other projects: while three of these are subordinate districts (“Rayons”) of Dushanbe, the 4th (Vakhsh) is nearby to Dushanbe in the Oblast of Khatlon (Figure 2).
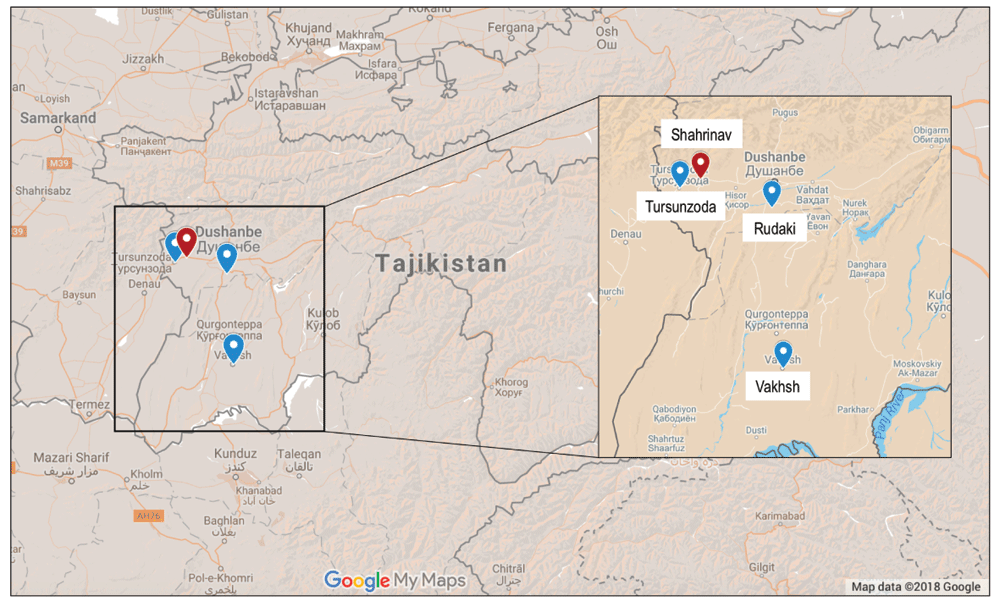
In order to provide more intensive support and ensure the quality of data collection, it was decided to focus on only one district. All PHC centres and the associated family doctors and nurses will be invited to take part in the training and pilot implementation. Shahrinav was chosen after considering its size, exposure to previous interventions, proximity to Dushanbe, year-round road access, and by recommendation from the Ministry of Health.
Within Shahrinav district, all PHCs were compared against the following exclusion criteria: (1) City Health Centre designation; (2) presence of narrow specialists (i.e. any physicians with specialization other than family medicine) within the clinic; (3) clinics with a family doctor to patient ratio of 1:4000 or more; or (4) clinics where information on the number of family doctors and/or family doctor to patient ratio is not available. If one or more of these four exclusion criteria were met, the clinic will not be included in the intervention or control arm.
Clinics allocated to the control arm will not receive the intervention; clinicians will proceed with usual care. The performance of individual health facilities will be compared from baseline to follow-up. To compare change in performance between intervention and control arms, the data from all intervention clinics will be aggregated together and compared to aggregated data of all control clinics.
Given the emphasis of hypertension management, including total CVD risk approach, indicators were developed to be calculated from a sample of hypertensive patients. These include indicators along the hypertension care pathway, including detection of hypertension, CVD risk assessment, risk factor modifying prescriptions, and BP control. Table 1 shows the primary and secondary indicators, the question the indicator seeks to answer, and the respective numerator and denominator definitions which will be used in the calculations.
Quantitative data collection tool. We developed a standardized data collection tool to collect the anonymized data required for calculation of each indicator (Table 2). This tool will also be digitized such that it can be used on a computer (offline) or smartphone (online). We estimate it take 15 minutes per unique health record in order to extract data. Since there is a lack of electricity and mobile phone reception in many of the clinics, it is likely that data will have to be extracted first on paper and then later input to a database using an online data form. Data extractors will be blinded to the allocation of health care facilities to intervention or control for baseline data collection, but blinding is not possible for follow-up data collection because after the intervention period, allocation will be apparent when data collectors visit clinics and look at patient records.
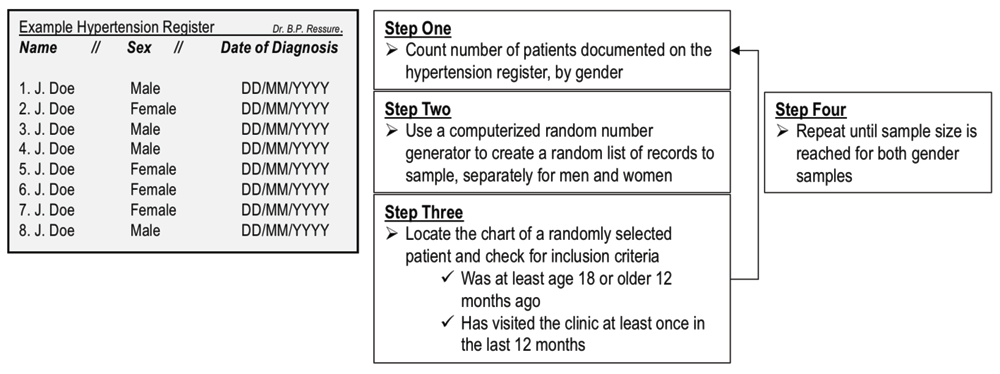
Method of randomly sampling patient records. Each family doctor owns and maintains a single register (list of patients) for hypertensive patients (henceforth “hypertension register”). The hypertension register will be used to randomly select patient records. A computerized random number generator will be used to randomly select patients from the register. The selected patient chart will then be found and checked to see if it meets two inclusion criteria: (1) the patient must have visited the clinic at least once in the previous 12 months; and (2) the patient must have been 18 years or older 12 months prior to the date of data selection. If both criteria are met, the record will be used for data extraction. If the record does not meet inclusion criteria, it will be returned and a new record will be randomly selected. This process will be repeated until the sample size for each family doctor has been met (Figure 3).
Sample size. A total of 400 patient records will be sampled at baseline and follow-up from each of the intervention and control arms (n=800). The sample is based on a power calculation for the primary indicator (proportion of hypertensive patients whose blood pressure is controlled), estimated based on a WHO report that the control level in low and middle income countries is generally about 20–25%13. A 10% point difference between the intervention and control arms could be detected having 310 to 350 observations in each group using 0.05 type I error rate and 0.2 type II error rate. Sampling will be stratified by gender and selected in a 1:1 ratio of men to women (200 men and 200 women per arm). In the event that there are too few patients of a given gender, patients from the opposite gender will be substituted.
Data analysis. The change in indicators from baseline to follow-up will be calculated. Subgroup analysis by age, gender, and other demographic features may be done as deemed appropriate.
Qualitative data collection.
Semi-structured interviews A purposive maximum variation sample of doctors and nurses from the intervention clinics will be invited to take part in semi-structured interviews at the end of the 12-month implementation phase. All participants will be required to provide written informed consent. Using an interview guide that will be adapted from previously published work21,22, interviews will be conducted one-on-one by members of the research team, audio recorded, and transcribed verbatim. Transcripts will be analysed thematically using framework thematic analysis, by two or more members of the research team, with oversight from an experienced qualitative researcher23.
Patient and public involvement Neither patients nor the public were involved in the methodological design.
We report methodology to adapt, pilot, and evaluate the WHO HEARTS technical package in a low-resource setting in a low-income country that can be used as an example for other jurisdictions with a high burden of cardiovascular diseases
Our methods focus on evaluating the effect of the WHO HEARTS interventions in a real-world health system and therefore are expected to yield practical information to inform the national scale up of essential interventions for non-communicable diseases
The sample size is based on one primary indicator and it is therefore possible that some of the secondary indicators will be statistically underpowered
Due to the practical and resource constraints, data collectors could not be blinded to follow-up data collection, and this could potentially bias the effect estimate
Although an important stakeholder in implementation, patient perspectives were not included in the evaluation design
Data collection has been completed and analysis is underway.
This project has been reviewed and granted ethical approval from the Republican Clinical Center for Family Medicine under the Ministry of Health and Social Protection of the Republic Tajikistan. As per the ethical approval review and the nature of this project, consent was not obtained from individual patients for their anonymized routine clinical data to be used.
Findings will be shared at the participant level (intervention clinics), national level through a national conference of key stakeholders, and internationally through publication in an open-access peer review journal. At the national level, the results will be used to assess the appropriateness and feasibility of national scale-up and mainstreaming into clinical practice. The raw data used in this project will not be made publicly available.
No data are associated with this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology of hypertension and other non-communicable diseases in Africa
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Sep 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)