Keywords
Brain derived neurotrophic factor, neurotrophin, BDNF, TrkB, p75NTR, synaptic plasticity, depressive disorders, memory, sortilin, SorCS2
Brain derived neurotrophic factor, neurotrophin, BDNF, TrkB, p75NTR, synaptic plasticity, depressive disorders, memory, sortilin, SorCS2
Neurotrophins are a small orthologous family of growth factors, consisting of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4) in mammals. Neurotrophins have a remarkably wide range of critically important functions in both neural and non-neural tissues, and this diversity of function is achieved by signaling mechanisms that are highly context dependent. This review will explore recent advances in understanding how such context-dependent signaling is achieved, focusing on two of the most extensively studied functions of neurotrophins: regulation of neuronal survival and axon and dendritic growth in the developing peripheral nervous system and regulation of synaptic function in the context of learning and memory in the adult central nervous system (CNS). However, the principles and mechanisms discussed will be relevant for understanding other neurotrophin functions as diverse as mediation of the effects of anti-depressant drugs1,2, regulation of cardiac development3, hypothalamic control of energy balance4 and oxytocin-dependent maternal behavior5, control of pancreatic glucose-induced insulin secretion6, and control of insulin-dependent glucose uptake7.
Two classes of receptors mediate neurotrophin effects. The 75 kDa neurotrophin receptor (p75NTR) binds all four neurotrophins, whereas orthologous members of the Trk gene family—TrkA, TrkB, and TrkC—are more selective. NGF and NT3 activate TrkA, BDNF and NT4 activate TrkB, and NT3 activates TrkC. Trk receptors are canonical receptor tyrosine kinases. p75NTR is a member (and indeed was the first-discovered member) of the tumor necrosis factor (TNF) receptor superfamily and the subclass of those receptors known as death receptors because they contain a cytoplasmic death domain that mediates key signaling effects8.
Further complexity in neurotrophin signaling results from the differential signaling capacity of mature neurotrophins and the longer precursor proteins (proneurotrophins) from which mature neurotrophins are released proteolytically. Proteolytic processing of proneurotrophins by prohormone convertases in the secretory pathway is not always complete, so proneurotrophins are sometimes secreted, and secreted neurotrophins may be variably released from proneurotrophins following secretion by membrane metalloproteinases. Both proneurotrophins and mature neurotrophins bind and activate p75NTR, whereas only mature neurotrophins can bind and activate Trk receptors9. Consequently, the extent of proteolytic processing of proneurotrophins, either in the secretory pathway or following secretion, determines the balance of p75NTR versus Trk signaling, a phenomenon that has received particular attention for BDNF/pro-BDNF signaling through p75NTR and TrkB at CNS synapses10. Remarkably, the cleaved pro-domain peptide of pro-BDNF has substantial stability and is stored in vesicles and co-secreted with BDNF11. This feature and the technical issue that antibodies against BDNF do not discriminate between pro-BDNF and mature BDNF, while antibodies against the pro-domain do not discriminate between pro-BDNF and its cleaved pro-domain peptide, have contributed to uncertainty and controversy concerning the forms in which BDNF is secreted. Remarkably, the pro-domain peptide itself may engage in p75NTR-dependent signaling12–14, although the physiological relevance of this phenomenon remains to be fully explored.
This review will explore recent findings concerning four themes that figure prominently in neurotrophin studies. The first two themes are explored below as separate topics, whereas the remaining two themes cut across all topics. Theme 1: in cells that express both p75NTR and Trk receptors, function of the two receptor systems is linked, but the two receptor systems often function oppositely. Theme 2: in the nervous system, neurotrophins may signal in an anterograde manner (from axon terminals to innervated targets), in a retrograde manner (from innervated target to innervating neuron), or in an autocrine manner and, in some systems, may use all three modes of action simultaneously. Theme 3: neurotrophin function is dependent on exquisitely controlled intracellular trafficking of neurotrophins and neurotrophin receptors. Theme 4: the nature of the signaling functions of p75NTR and Trk receptors in neurons differs in different cellular contexts. The modes of signaling may differ in different types of neurons, at different stages of development of a neuron, and in somatic, dendritic, and axonal cell compartments of neurons.
That p75NTR and Trk receptors often function oppositely has been referred to as the yin and yang of neurotrophin action15. Developing sympathetic neurons, for example, express both TrkA and p75NTR. For these neurons, activation of TrkA by NT3 promotes axon growth, and activation of TrkA by NGF promotes axon growth and neuronal survival16. On the other hand, activation of p75NTR by BDNF/pro-BDNF promotes axonal pruning and cell death17,18. In the adult CNS, BDNF-dependent activation of TrkB strengthens synaptic function and promotes long-term potentiation whereas BDNF-dependent activation of p75NTR weakens synaptic function and promotes long-term depression15. However, seemingly, nothing is ever simple in neurotrophin biology, as, under some circumstances, p75NTR signaling can promote neuronal survival19 and Trk signaling can promote neuronal cell death20,21 (discussed more fully below).
Functions of p75NTR and TrkB are influenced by two co-receptors—sortilin and SorCS2—membrane proteins that are members of the VPS10p-domain protein family. The preferential interaction of proneurotrophins with p75NTR is dependent on association of p75NTR with sortilin or SorCS29,22, which make binding contacts with the neurotrophin pro-domains23. Such interactions also mediate sortilin-dependent intracellular sorting of pro-BDNF into the regulated secretory pathway24 and mediate p75NTR-dependent disassembly of hippocampal dendritic spines elicited by secreted cleaved BDNF pro-domain12. In sensory neurons of the peripheral nervous system, association of sortilin with TrkA, TrkB, or TrkC promotes anterograde transport of these receptors to their axon terminal sites of action25. In dendrites of hippocampal neurons, BDNF-dependent activation of TrkB required for dendritic spine formation and induction of synaptic long-term potentiation requires association of TrkB with SorCS2 whereas SorCS2 association with p75NTR is required for pro-BDNF-dependent induction of synaptic long-term depression26. Evidence suggests that sortilin or SorCS2 forms ternary complexes with neurotrophins or proneurotrophins and p75NTR or Trk receptors22,23,27. Figure 1 illustrates a speculative model for the nature of such ternary complexes.
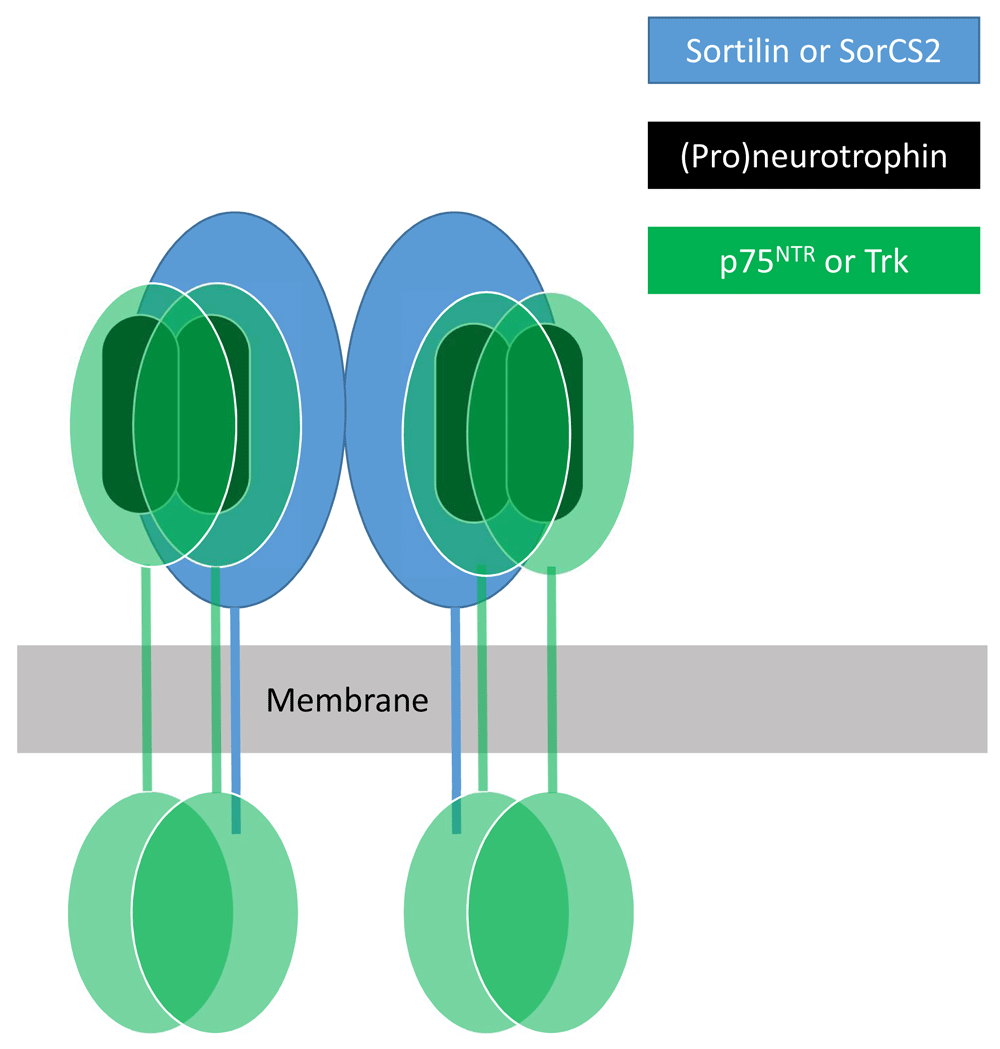
At least two members of the VPS10p-domain family of membrane proteins—sortilin and SorCS2—bind neurotrophins and their p75NTR and Trk receptors. Neurotrophins bind as dimers to dimeric forms of p75NTR and Trks. SorCS2 exists as a dimer, each subunit of which can apparently engage a neurotrophin (or proneurotrophin) dimer and associated dimeric p75NTR or Trk receptor18.
The first function ascribed to neurotrophins historically, regulation of neuronal survival by factors released by innervated targets, requires that neurotrophin receptors be localized to the axon terminus and, where the innervated targets are neurons, implies that neurotrophins are released from dendritic sites. On the other hand, for more recently characterized systems in which neurotrophins regulate synaptic function rather than neuronal survival, it is often initially unclear whether neurotrophins are released from dendrites to activate axonal receptors or whether neurotrophins are released from axons to activate dendritic receptors. Remarkably, not only do all of these scenarios come into play but a third scenario involving autocrine release and receptor activation in the same cell compartment plays an important role, as discussed below. Exquisitely complex mechanisms create ordered behavior from a potentially chaotic system by controlling neurotrophin release and receptor localization. I will summarize recent studies shedding light on these processes.
In circumstances where neurotrophins secreted by the innervated target control neuronal survival, how does activation of receptors at the axon terminus influence required changes in nuclear gene expression when the somatic compartment containing the nucleus may be many centimeters distant from the axon terminus? An abundance of evidence indicates that a major mechanism mediating this effect is neurotrophin-dependent endocytic internalization of neurotrophin/Trk complexes at the axon terminus, followed by retrograde axonal transport of activated receptors in the vesicular complex28–31. This critically important transport of Trk receptors from axon terminus to soma must be balanced by a similar rate of transport of receptors translated in the soma to the axon terminus. For TrkA, one remarkable mechanism achieving this balance directly links arrival in the soma of retrogradely transported vesicles containing activated TrkA to anterograde transport of nascent TrkA. Nascent TrkA receptors are inserted into somatodendritic membranes and then endocytically internalized before anterograde axonal transport to the axon terminus, a process known as transcytosis. Endocytosis of nascent TrkA from somatodendritic membranes to initiate anterograde transport is triggered by tyrosine phosphorylation of TrkA by activated TrkA arriving through retrograde axonal transport32. It is worth noting, however, that retrograde axonal transport is not the only mechanism that can deliver activated Trk receptors to the somatodendritic compartment. It is noteworthy that a transactivation mechanism causes activity of several G-protein-coupled receptors (GPCRs), including PACAP (pituitary adenylate cyclase-activating peptide) and adenosine receptors, to activate Trk receptors in the somatodendritic compartment33. Therefore, it is interesting to speculate that GPCR signaling might enhance the accumulation of Trk receptors at axonal termini, rendering the capacity for retrograde signaling dependent on the cellular context.
Although retrograde axonal transport links activation of Trk receptors at the axon terminus to neuronal survival, activated Trk receptors acting locally contribute to the regulation of growth and arborization of the axon terminus, and mechanisms exist to segregate local and retrograde signaling modalities. For example, during sympathetic neuronal development, NT3 released along the path of axon growth sustains axonal elongation by activation of TrkA in a manner that does not require retrograde signaling to the soma, but NT3 is insufficient to sustain neuronal survival. Instead, activation of TrkA by NGF encountered when the axon reaches its final target sustains neuronal survival by retrograde signaling to the soma. How do NT3 and NGF engaging the same TrkA receptor signal locally in one case and at a distance in the other? One elegantly simple mechanism results from the stability of NGF/TrkA binding at the acidic pH of endosomes. Unlike NGF, NT3 dissociates from TrkA at acidic pH and cannot maintain the sustained activation of internalized TrkA required to convey signals to the cell soma34. Additionally, a molecular switch mechanism causes NGF activation of TrkA to suppress subsequent activation of TrkA by NT3. This mechanism involves NGF/TrkA retrograde signaling, which transcriptionally upregulates Coronin-1. Coronin-1 suppresses the ability of NT3/TrkA complexes to release calcium ion from intracellular stores in the axonal compartment. In the absence of Coronin-1, sympathetic axons overshoot their targets35.
In sympathetic neurons, association with TrkA with an axonally enriched non-translated transcript, Tp53inp2, is required for the NGF-dependent TrkA endocytosis event necessary to initiate retrograde axonal signaling supporting axon growth and cell survival36. Whether similar mechanisms exist for TrkB and TrkC remains unknown. Sympathetic neurons express p75NTR as well as TrkA, and, like TrkA, p75NTR mediates both local functions at the axon terminus and functions in the cell soma requiring retrograde axonal signaling. Several mechanisms link functions of p75NTR and TrkA. p75NTR exhibits two distinct modes of signaling. Association of neurotrophins with p75NTR dimers at the cell surface causes a conformation change of the receptors’ intracellular domains oppositely modulating signaling adapter proteins linking p75NTR to regulation of the RhoA pathway and nuclear factor kappa B (NF-κB) and c-Jun N-terminal kinase (JNK) pathways37. However, in sympathetic neurons, an alternative mode of p75NTR signaling, leading to nuclear accumulation of NRIF (nuclear receptor-interacting factor) and cell death, involves γ-secretase-mediated cytoplasmic release of the intracellular domain (ICD) of p75NTR, which is promoted by neurotrophin-dependent activation of Trk receptors but is not directly promoted by neurotrophin binding p75NTR38–40. p75NTR signaling alternatively can activate cell survival-promoting pathways, such as the NF-κB pathway, or cell death-inducing pathways, such as the JNK pathway, and mechanisms determining these alternative responses are poorly understood. Modulation of γ-secretase-mediated release of the p75NTR ICD fragment is one mechanism that may govern these responses, as pharmacologically blocking cytoplasmic release of the ICD fragment abolishes NF-κB activation and allows JNK-dependent induction of cell death in cerebellar granule cell neurons19.
In addition to the Trk-dependent release of the p75NTR ICD, several other mechanisms link Trk and p75NTR signaling. Association of p75NTR with TrkA increases the affinity of TrkA for NGF and blunts activation of TrkA by NT341,42. In sympathetic neurons, NGF-dependent activation of TrkA leading to Arf6 activation promotes trafficking of p75NTR from intracellular vesicular stores to the cell surface. In contrast, NT3 activation of TrkA does not promote Arf6 activation and p75NTR trafficking. Thus, NGF/TrkA signaling suppresses NT3/TrkA signaling, sharpening the developmental switch from NT3- to NGF-dependent functions43. p75NTR signaling at the axon terminus can locally regulate axonal growth cone collapse. However, p75NTR also can signal retrogradely from the axon terminus to the soma to induce neuronal cell death. Although Trk activation promotes release of the p75NTR ICD, loss of NGF-dependent TrkA activation in sympathetic neurons causes enhanced production of the ICD fragment and HDAC1-dependent retrograde trafficking of this fragment mediates retrograde axonal conveyance of the p75NTR cell death signal44. Similarly, enhanced release of the p75NTR ICD fragment has been implicated as a mechanism responsible for neuronal cell death promoted by unliganded TrkA and TrkC in the developing peripheral nervous system20.
Although all the neurotrophins and neurotrophin receptors function in the CNS, the most broadly important neurotrophin in the brain is BDNF, which influences many types of neural plasticity in diverse neuronal populations, including cortical and hippocampal neurons, through TrkB and p75NTR receptors. BDNF function in the hippocampus, and particularly at the synapses of CA3 Schaffer collaterals on CA1 dendrites, has been extensively studied because BDNF interactions with TrkB and p75TR at hippocampal synapses are critical for long-term potentiation45,46, an experimental synaptic model for learning and memory. Unraveling these mechanisms is far from straightforward, as BDNF can be released by both pre-synaptic axon termini and post-synaptic dendrites, and the receptors are present both pre-synaptically and post-synaptically47. Furthermore, peri-synaptic microglia, astrocytes, and oligodendroglia are all physiologically relevant sources of BDNF release at CNS synapses48–50. Although both pre-synaptic and post-synaptic action of BDNF has been described, a particularly thorough report from 2016 describes an autocrine mode of BDNF/TrkB signaling in CA1 dendritic spines involving NMDA receptor-dependent activation of CaMKII-dependent BDNF release51.
Studies in Aplysia, an important model organism for the study of mechanisms of learning and memory, have revealed an important role for pre-synaptic autocrine neurotrophin action. Aplysia has a single neurotrophin gene (ApNT) and a single Trk receptor gene (ApTrk)52. ApNT promotes 5HT-mediated consolidation of learning-related facilitation of a sensory neuron/motor neuron synapse. 5HT-dependent production of cAMP pre-synaptically promotes pre-synaptic release of ApNT, which activates pre-synaptic ApTrk driving phospholipase C-dependent calcium ion release, resulting in further elevation of cAMP by a calcium-dependent adenylate cyclase. This constitutes a positive feedback loop driving ApNT release and neurotransmitter (glutamate) release53. Pre-synaptically released ApNT acting on post-synaptic ApTrk promotes post-synaptic ApNT release, which acts pre-synaptically. Thus, two neurotrophin feed-forward systems provide signal amplification for 5HT’s effects on synaptic function54.
An important feature of BDNF function in mammalian dendrites is that dendritic BDNF is a product of peri-synaptic protein synthesis. BDNF transcription employs nine different promoters which, in concert with alternative splicing of 5′ untranslated regions (5′ UTRs), generate 22 different transcripts all encoding identical proteins55. In vitro studies reveal that these 5′ UTRs differentially direct different transcripts to different subcellular domains56. The differential regulation of the different promoters by neural activity, coupled with the different subcellular targeting of the different transcripts, allows complex regulation of neuronal responses. For example, transgenic mouse models reveal that different splice variants differentially control size of spines on apical versus basal CA1 dendrites57. In concert with BDNF/TrkB action promoting the formation of hippocampal dendritic spines, pro-BDNF/p75NTR action suppresses the formation of dendritic spines58. As noted above, SorCS2 acts as a necessary co-receptor for both p75NTR and TrkB acting oppositely to control the formation of hippocampal dendritic spines. Whether the differential engagement of p75NTR versus TrkB by SorCS2 is an important point of regulation is a question that deserves investigation.
Despite the impressive recent progress in revealing the extraordinary complexity of neurotrophin signaling functions, many unanswered questions remain. The manner by which neurotrophin-deprived Trk receptors promote the release of the proapoptotic p75NTR ICD fragment remains mysterious, and the relative importance of signaling by intact cell-surface p75NTR versus signaling by the cleaved p75NTR ICD fragment in various contexts remains unclear. Indeed, many aspects of p75/Trk functional interactions remain unclear despite decades of study. In many cases, mechanisms governing neurotrophin receptor anterograde and retrograde trafficking have been characterized for a single Trk paralog and in a single neuronal cell type at a particular stage of development. It should be a priority to determine the extent to which such mechanisms apply to other Trk paralogs and in other neuronal cell types or other stages of development.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 19 Sep 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)