Keywords
oxidative stress, bleach, polyphosphate, bacteria, protein folding, chaperone, holdase, chlorination
oxidative stress, bleach, polyphosphate, bacteria, protein folding, chaperone, holdase, chlorination
Like all aerobic organisms, bacteria naturally produce reactive oxygen species (ROS) as metabolic by-products, for instance during electron transfer in the respiratory chain. The addition of one electron to O2 leads to the production of the superoxide radical (O2•–), a toxic compound, which dismutates to form hydrogen peroxide (H2O2) and molecular oxygen (O2) either spontaneously or via catalysis by superoxide dismutases1–3. H2O2 can then react with ferrous iron to generate more reactive hydroxyl radicals (•OH) by the Fenton reaction. These oxidizing molecules can damage cellular components including DNA, membrane lipids, and proteins, which can lead to cell death. Therefore, bacteria have evolved defense mechanisms, which include enzymes, such as catalases and peroxiredoxins, that directly react with ROS to convert them to harmless products, and repair enzymes, such as thioredoxins and methionine sulfoxide reductases, that catalyze the reduction of oxidized amino acids in damaged proteins. For more information on the mechanisms that allow bacteria to cope with oxidants and rescue oxidatively damaged proteins, we refer the reader to a recent review in which we discuss the role of the thioredoxin and glutaredoxin systems and highlight the importance of protein repair in bacterial physiology and virulence4.
Because of their toxicity, it is not surprising that the immune system of multicellular eukaryotes uses ROS as weapons to kill bacteria. When bacteria enter a tissue, the inflammatory response is turned on and phagocytes (neutrophils and macrophages) are recruited to the site of infection5. These cells, whose cytoplasm is filled with lysosomal granules containing a variety of bactericidal and digestive enzymes6,7, are able to engulf bacteria. After phagocytosis, the phagosome and the granules fuse, forming a phagolysosome6,7. Then, high levels of ROS (O2 •– and H2O2) are produced in a phenomenon known as “oxidative burst”6–8, strongly contributing to the killing of the bacterium.
In neutrophils, ROS production induces the release of myeloperoxidase (MPO), a glycoprotein stored in the phagocyte granules, into the phagolysosome. This enzyme converts H2O2 and chloride into hypochlorous acid (HOCl)5, a strong oxidant (E0’ [HOCl/Cl–] = 1.28 V) that is also the active ingredient of household bleach, the most widely used disinfectant. HOCl is extremely effective and reacts with most macromolecules, including lipids, cholesterol, NADH, nucleotides, and proteins9–11. In contrast to H2O2, which can diffuse through membranes12 and has a substantially longer lifetime (10 µs;13), HOCl acts rapidly and locally, with a lifetime of ~0.1 µs14 and a short diffusion length in vivo (0.03 µm when it reacts with cysteines and methionines15). Thus, by catalyzing the conversion of long-lived, diffusible H2O2 into locally confined HOCl, MPO contributes to the prevention of collateral tissue damage during oxidative burst, allowing the specific targeting of the engulfed bacterial pathogen14.
Although HOCl targets all cellular components, proteins, because of their reactivity and high abundance, are thought to be its primary target. The oxidation of amino acid side-chains in proteins (Figure 1) can cause the loss of secondary or tertiary structure, thereby impacting protein stability and activity. HOCl reacts extremely quickly (k≈ 3 × 107 M–1. s–1) with sulfur-containing residues (cysteines and methionines)10,11,16. Cysteine thiols are first rapidly chlorinated to form a sulfenyl chloride, an unstable intermediate that can react with water to form a sulfenic acid (R-SOH) (Figure 1). Most sulfenic acids are highly unstable (half-life in minutes17) and either react with a cysteine thiol present in the vicinity to form a disulfide, whose formation is in principle reversible by the action of an oxidoreductase like thioredoxin18, or are further oxidized to sulfinic (R-SO2H) and sulfonic (R-SO3H) acids (Figure 1), two irreversible modifications that typically cause protein inactivation and degradation. Degrossoli and co-workers showed that exposure of bacteria to the oxidant mixture released during phagocytosis causes a rapid and massive oxidation of thiols19,20. By taking advantage of fluorescent redox-sensitive protein probes expressed by the engulfed bacteria, they highlighted the critical role of MPO-generated HOCl in the toxic oxidizing cocktail released by immune cells19.
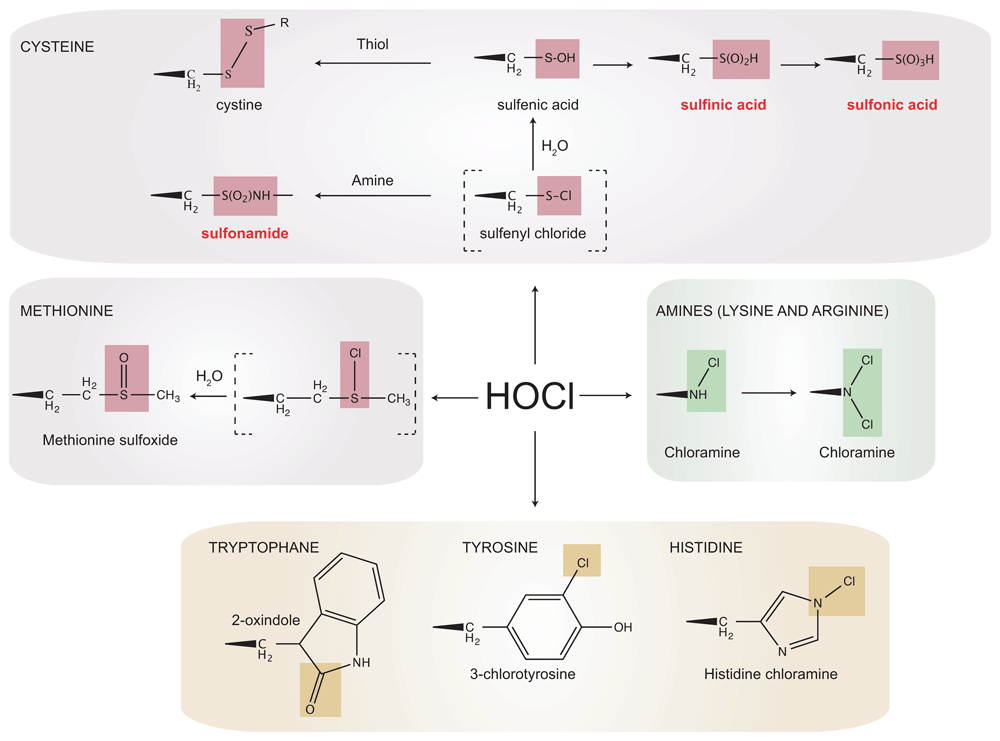
HOCl modifies the side-chains of several amino acids. Reaction with the thiol group of cysteine residues leads to the formation of an unstable sulfenyl chloride. Sulfenyl chloride quickly reacts with water to form a sulfenic acid or with primary and secondary amines to form sulfonamide crosslinks, which are irreversible. Sulfenic acids can be reduced back to a thiol by the cytoplasmic reducing systems, be further oxidized to sulfinic or sulfonic acids that are irreversible and lead to protein inactivation and degradation, or react with another thiol to form disulfide bonds. Irreversibly oxidized forms are indicated in red. HOCl also reacts with methionine residues to form methionine sulfoxides. Primary and secondary amines (lysine and arginine) are the second targets of HOCl in proteins, which chlorinates them to form chloramines (the secondary amine of arginine is not shown). The imidazole ring of histidine reacts with HOCl to form a short-lived chloramine, which rapidly transfers its chlorine group to another amine. Tryptophan reacts with HOCl to form 2-oxindole while reaction of HOCl with tyrosine forms 3-chlorotyrosine.
Methionines can be oxidized to methionine sulfoxides (Met-SO), and this oxidation is likely to play a critical role in the bactericidal action of HOCl, as strains lacking methionine sulfoxide reductases, enzymes that reduce methionine sulfoxides back to methionine, become more sensitive to HOCl21. In line with this idea, we recently identified an enzymatic system expressed in the cell envelope of Gram-negative bacteria that participates in the defense mechanisms against HOCl by reducing oxidized methionine residues in this compartment22. This system involves the molybdenum-containing enzyme MsrP and the heme-binding membrane protein MsrQ and uses electrons from the respiratory chain for methionine rescue. Remarkably, MsrP and MsrQ are specifically induced by HOCl in Escherichia coli, and not by H2O2, which further highlights the physiological need for cellular systems devoted to the defense against HOCl22.
In addition to sulfur-containing residues, primary (Figure 1) and secondary amines (not shown) are also susceptible to HOCl, which chlorinates them to form chloramines (k≈ 103–105 M–1. s–1)10,11,16. Tryptophan is also thought to react with HOCl to form 2-oxindole, but how these molecules form remains unclear (Figure 1)10,11. The imidazole ring of histidine reacts with HOCl to form a short-lived chloramine, which rapidly transfers its chlorine group to another amine. Finally, the chlorination of tyrosine into 3-chlorotyrosine is a marker used to detect HOCl-induced damage (Figure 1)10,11.
The mechanism by which HOCl contributes to bacterial killing in the phagolysosome is not fully understood5. However, it is thought to be a combination of events including oxidation-induced protein aggregation23 and a drastic decrease in cellular ATP caused by the inactivation of the F1-ATP synthase, loss of glucose respiration, and the formation of polyphosphate (PolyP)24. The bactericidal activity of HOCl can also be explained by the loss of activity of GroEL (Hsp60), an essential chaperone inactivated upon HOCl treatment25,26.
In the last decade, important insights into the mechanisms used by bacteria to mount effective, often complex responses against HOCl have been obtained. For instance, transcription factors that specifically respond to HOCl have been described in E. coli and other bacteria27. They include HypT, which is activated through methionine oxidation28,29, and NemR, which is activated via cysteine oxidation. Furthermore, three HOCl-activated chaperones have been identified and shown to be important during HOCl stress. These chaperones are ATP-independent holdases, i.e. chaperones that prevent protein aggregation by binding unfolded proteins but do not promote protein refolding, and thus function during HOCl stress, when the ATP-dependent foldases, i.e. chaperones actively promoting protein refolding, are inactive (Figure 2). In the following sections, we will briefly describe HOCl-activated chaperones and explain how they are activated under conditions that inactivate most other proteins30.
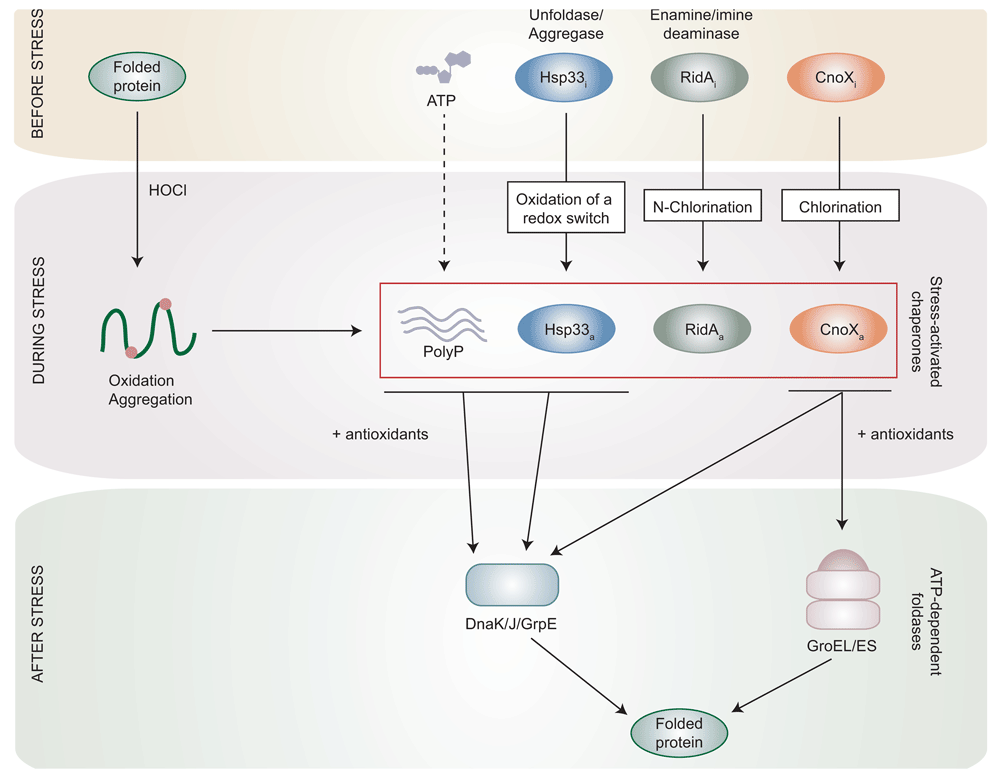
Upon HOCl stress, most proteins become oxidized and lose their three-dimensional structure, ultimately leading to their aggregation. In parallel, the oxidation or chlorination of stress-induced holdases (Hsp33, RidA, and CnoX) activates them upon HOCl stress, which allows them to bind and protect their substrates. Polyphosphate (PolyP), a chemical chaperone synthesized from ATP, has also been shown to bind unfolded proteins during stress. After stress, when the ATP pool is replenished and oxidative stress relieved, these stress-induced holdases cooperate with antioxidants to transfer their substrates to either DnaK/J/GrpE or GroEL/ES for proper refolding.
The first HOCl-activated chaperone identified was Hsp33, a protein which was recently described to work, under normal conditions, as an unfoldase/aggregase transferring EF-Tu to the Lon protease for degradation31. However, when exposed to HOCl, Hsp33 is quickly transformed into a holdase through the oxidation of a redox switch involving four conserved, zinc-binding cysteine residues32–38. Oxidation of this redox switch induces structural changes in Hsp33 that now exposes hydrophobic surfaces and can interact with unfolded proteins32–38. Upon the cell’s return to normal conditions, oxidoreductases reduce Hsp33’s redox switch before its substrates are shifted to the ATP-dependent foldase DnaK/J/GrpE for refolding39,40 (Figure 2).
Another HOCl-activated chaperone is the E. coli protein RidA, for which the chaperone activity has been mostly studied in vitro41. Interestingly, RidA, which normally functions as an enamine/imine deaminase involved in the synthesis of branched-chain amino acids42, loses its deaminase activity when incubated with HOCl while it turns into a holdase via the reversible N-chlorination of positively charged residues, an unprecedented post-translational modification. N-chlorination makes the surface of RidA more hydrophobic, which activates its holdase activity41 (Figure 2). The fact that ridA mutant cells are more sensitive to HOCl41 suggests that RidA protects E. coli against HOCl-induced damage. However, further investigation is required to determine the functional relevance of the HOCl-induced chaperone activity of this protein in vivo and its potential role in the proteostasis network under HOCl stress.
We recently identified CnoX as a novel type of protein folding factor that is essential for cell survival when E. coli is exposed to HOCl43. We demonstrated that HOCl turns CnoX into a powerful holdase by chlorination in a mechanism similar to that described for RidA41. Remarkably, CnoX can both function as a holdase and form mixed-disulfide complexes with client proteins. Under the latter role, CnoX prevents sensitive cysteine residues in its substrates from being irreversibly oxidized, which could otherwise have a detrimental effect on refolding and/or block reactivation. Because CnoX can solve two problems faced by proteins (aggregation and overoxidation), it has become the first member of a new class of proteins: the chaperedoxins43. Importantly, we established that, after stress, CnoX is capable of transferring its substrates not only to DnaK/J/GrpE, like Hsp3339, but also to GroEL/ES, the only chaperone system essential for E. coli growth and survival44. This feature is conserved in the Caulobacter crescentus CnoX homologue45 (Figure 2). CnoX is, to our knowledge, the first holdase shown to cooperate with GroEL/ES for protein refolding.
In addition to the proteins described above, work from the Jakob laboratory has led to the identification of PolyP, an inorganic polymer synthesized from ATP, as a chemical chaperone able to stabilize proteins during HOCl stress46 (Figure 2). Accordingly, intracellular levels of PolyP increase during HOCl stress, as a result of both decreased hydrolysis46 and probably also increased synthesis, although this remains to be firmly established.
Whereas the important role for reducing enzymes, such as catalases, peroxiredoxins, thioredoxins, and glutaredoxins, in fighting oxidative stress in bacteria has been known for some time, the crucial function of HOCl-induced chaperones for proteostasis has emerged more recently. The identification of an increasing number of these chaperones, in both prokaryotes and eukaryotes, raises a number of questions and hypotheses that will have to be addressed in the future. First, because activation by chlorination appears to be rather unspecific compared to activation by oxidation of cysteine residues, like in Hsp33, it is likely that additional proteins share the ability to be activated by HOCl. Supporting this, it was recently reported that a number of proteins from human blood plasma are converted into holdases by HOCl via N-chlorination47. Second, the identified stress-induced chaperones are expressed under non-stress conditions and are conserved in a large number of organisms, including non-pathogenic bacteria that are less likely to be exposed to high levels of HOCl in their natural environment. It is therefore tempting to speculate that these proteins display a basal function under normal conditions but evolved in certain organisms to act as chaperones under specific stress conditions. Focusing on the CnoX chaperedoxin expressed by the aquatic bacterium C. crescentus, we recently found that, in contrast to its E. coli counterpart, it functions as a thioredoxin and a constitutive holdase that does not need to be activated by HOCl. Thus, within the family of CnoX proteins, only certain proteins (such as E. coli CnoX) have evolved to provide specific protection against HOCl stress45. In the same line, it was recently shown that N-chlorination does not activate the homolog of RidA from Staphylococcus aureus into a chaperone48. Thus, future work should determine the extent of the stress-induced chaperone network upon HOCl stress as well as the roles for these proteins under non-stress conditions and/or in non-pathogenic organisms.
H2O2, hydrogen peroxide; HOCl, hypochlorous acid; O2•–, superoxide radical; O2, molecular oxygen; •OH, hydroxyl radical; MPO, myeloperoxidase; PolyP, polyphosphate; ROS, reactive oxygen species.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 23 Sep 19 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)