Keywords
Ancorina sp., cytotoxicity, apoptosis, caspase-3, breast cancer
Ancorina sp., cytotoxicity, apoptosis, caspase-3, breast cancer
The revised version includes edited methods and figures. As suggested, I added more detail in the methods section, especially regarding the extraction and cytotoxicity assay. New figures 1 & 2 now show more information, making the data clearer and easier to understand.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Breast cancer is the second leading cause of death in women after cervical cancer. In 2016 breast cancer cases have occurred in 40 per 100,000 women in Indonesia1. Medical treatment for breast cancer is currently widely applied2. However, medical treatment can cause side effects, namely the death of healthy cells surrounding cancer cells3. Alternative methods of breast cancer treatment with reduced side effects are needed, such as treatments using natural anticancer agents3.
There are many cancer treatment methods such as anti-angiogenesis therapy4, cell cycle inhibitors5, and photodynamic therapy6. Induction of apoptosis is the most common approach in cancer therapy because apoptosis has specific abilities to kill certain cells7. One characteristic of cancer cell is loss of ability for apoptosis8. The ability of apoptosis to kill abnormal cells can prevent the occurrence of cancer growth9. Induction of apoptosis occurs through three apoptotic-signaling pathways:extrinsic, intrinsic and perforin/granzyme pathways. Apoptosis path activation is marked by the activation of caspases. Caspase is found in normal cells as an inactive zymogen (procaspase). Active caspase activates other caspases, forming the ‘caspase cascade’. Activation of caspase 8 and 9 will cause activation of caspase-3 as a downstream effector, which induces apoptosis10.
Previous studies found many natural apoptosis-inducing compounds isolated from marine sponge that can be developed as natural medicine11. Fraction of Negombata magnifica sponge is able to induce apoptosis in hepatocellular carcinoma12. Sponge extract of Haliclona sp. able to increase the percentage of apoptosis and significantly increase the expression of apoptotic gene p53, p21, caspase-8, and caspase-3 in A549 lung cancer cells13.
Natural anticancer agents are usually extracted by a particular solvent. Different solvents cause different effects on the disease. Some previous researchers have isolated sponge bioactive compounds using both polar and non-polar solvents. For example, cytotoxic compounds have been successfully isolated from sponge Dactylospongia elegans and Pachychalina alcaloidifera using methanol14,15. Organic compounds have been successfully isolated from the sponge Condrosia reniformes, Tethya rubra, Tethya ignis, Mycale angulosa and Dysidea avara as a drug therapy for Chagas disease using acetone solvents16. Terpenoids have been successfully isolated from sponge Iricina sp. and Spongia sp. using ethanol solvent17. Anticancer compounds have been successfully isolated from Petrosia sp., Jaspis sp. and heterogeneous Pericharax using dichloromethane:methanol (1:1)18. Some studies also mention that sponge bioactive compounds, antiviral, antimicrobial, antifungal, and anticancer compounds, have been successfully isolated with methanol19–21, ethanol22, dichloromethane and combination of dichloromethane:methanol (1:1)23–26.
The objective of this study is to determine the cytotoxicity of Ancorina sp. extract in breast cancer T47D cells and measure extract-induced apoptosis through activation of caspase-3. In this study we use three solvents: methanol (polar), dichloromethane (non-polar) and mixture of both solvents to determine the most effective solvent. Furthermore this study used T47D cells as a model for breast cancer cells because T47D cells are able to express caspase-3, which is an effector of apoptotic induction27.
Ancorina sp. were collected from Wedi Ombo Beach, Gunungkidul, Yogyakarta, Indonesia. Samples were washed to remove debris and residual salt. Samples were transferred to the laboratory in methanol, dichloromethane and dichloromethane:methanol (1:1) under cool condition.
Fresh samples were crushed in a blender in methanol, dichloromethane and dichloromethane methanol (1:1) then macerated for 24 hours. The samples were filtered using whatman no 1 (Sigma) and the residue was re-extracted for two times. The total filtrate was then naturally air drying in room temperature to obtain crude extract paste.
We used T47D cells obtained from Integrated Laboratory of Research and Testing, Universitas Gadjah Mada (LPPT UGM).
The cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 2% penicillin streptomycin and 0.5% Fungizone. Cells were harvested after reaching 80% confluence using 0.25% Trypsin-EDTA. Cells were cultured in 96-well microplates (1 × 103 cells/well) in 100 μL RPMI and incubated at 37°C with 5% CO2 overnight.
Doxorubicin at 5 µg/mL was used as the positive control whereas T47D cells cultured in medium was used as the negative control and cells cultured in 0.5% DMSO in medium was used as the solvent blank.
Cytotoxicity was assessed using the MTT assay. After the cells were incubated for 24 h with the serial dilution 15.68, 31.25, 62.50, 125 to 250 µg/mL of extract, 0.5% MTT solution was added and the cells were incubated for 4 h followed by addition of stopper reagent (10% SDS in 0.1 N HCl). Each treatment was subjected with 3 replication. Those serial concentration is based on preliminary experiments. The optical density (OD) was measured at 550 nm using Microplate Reader BIO-RAD 680XR. The percentage of cell viability was obtained by this formula:
Inhibitory Concentration 50% (IC50) of Ancorina sp. was then determined by probit analysis using value among cell viability and log concentration of extracts. IC50 of each extract is used for FACS experiment.
Briefly, T47D cells were seeded in 6-well microplates in 3×103μL RPMI. In total, 1×106 cells were treated by IC50 concentrations of three extracts or doxorubicin for 24 h. Cells were stained by Annexin V-PI Biolegend for apoptosis test and by BD Cytofix / Cytoperm™ for caspase-3 activation test. The sample was measured using flow cytometer BD FACSCalibur™. Flowcytometry output by BD FACSCalibur™ was shown in four quadrants. The first quadrant contains normal living cells population that respond negatively to Annexin V-FITC and propidium iodide (PI). Second quadrant contains early apoptotic cells populations that respond positively to Annexin V-FITC. Third quadrant contains the late apoptotic cells population which responds positively to Annexin V-FITC and Propidium Iodide (PI). Whereas, the fourth quadrant contains a population of necrotic cells that respond negatively to Annexin V-FITC and respond positively to PI28. On the other hand, in the caspase-3 test the black area indicated control cells while R1 showed caspase-3 activated cells.
The cell viability of T47D cells after methanol, dichloromethane and dichloromethane: methanol (1:1) extracts treatment are presented in Figure 1. The concentration of extracts reduced the viability of investigated cells by 50% (IC50), which has been reported in Table 1.
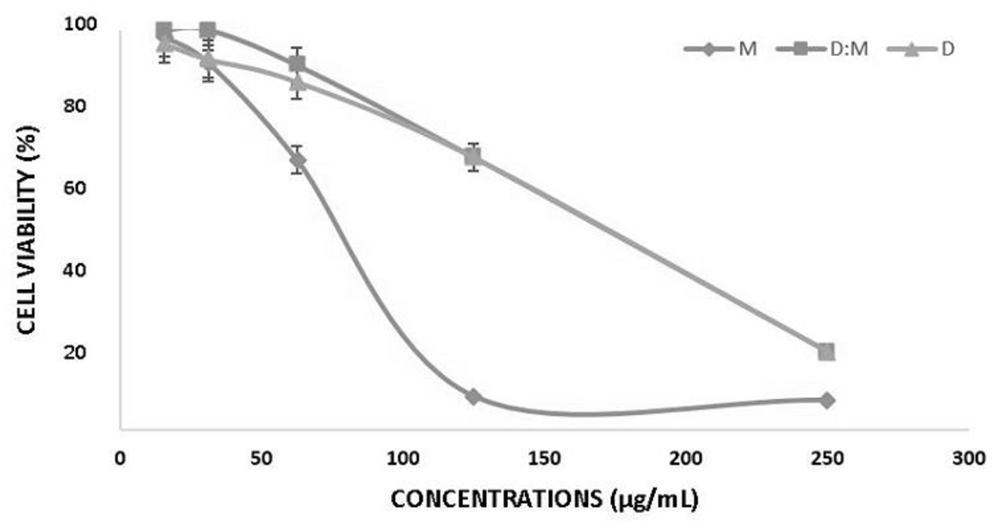
Methanol (M), Dichloromethane:Methanol (D:M) and Dichloromethane (D). Error bar shows standard deviation.
| Treatments | IC50 value (µg/mL) |
|---|---|
| Methanol | 84.25a ± 9.52 |
| Dichloromethane:methanol (1:1) | 121.45b ± 10.11 |
| Dichloromethane | 99.85ab ± 11.79 |
All Ancorina sp. extracts inhibited the proliferation of cancer cells in a dose dependent manner. The higher concentration of extract caused the lower percentage of T47D cell viability. All extracts were cytotoxic to T47D cells. IC50 value of methanol was significantly different to dichloromethane:methanol but wasn’t significantly different to dichloromethane.
We analyzed cell death qualitatively by examining cell morphological change and quantitatively by flow cytometry using Annexin-V after 24 h incubation of extracts.
The DMSO treated cell and control showed living cells withnormal morphology. T47D cells in these groups form tightly cohesive mass structures displaying robust cell-cell adhesions. However, after doxorubicin and extract treatment most cells undergo death. The cells became shrunken and showed signs of detachment from the surface of the wells, which denoted cell death
Cell morphology after treatment can be seen in Figure 2. Morphology of T47D cell showed that methanol extract caused most cells population to undergo death (approximately more than 70%), while dichloromethane extract resulted in almost half cell population deaths. The combination of methanol and dichloromethane (1:1) extract causes fewer cell deaths (<50%). This data supports the cytotoxicity assay that the Ancorina sp. extracts successfully induced cell death
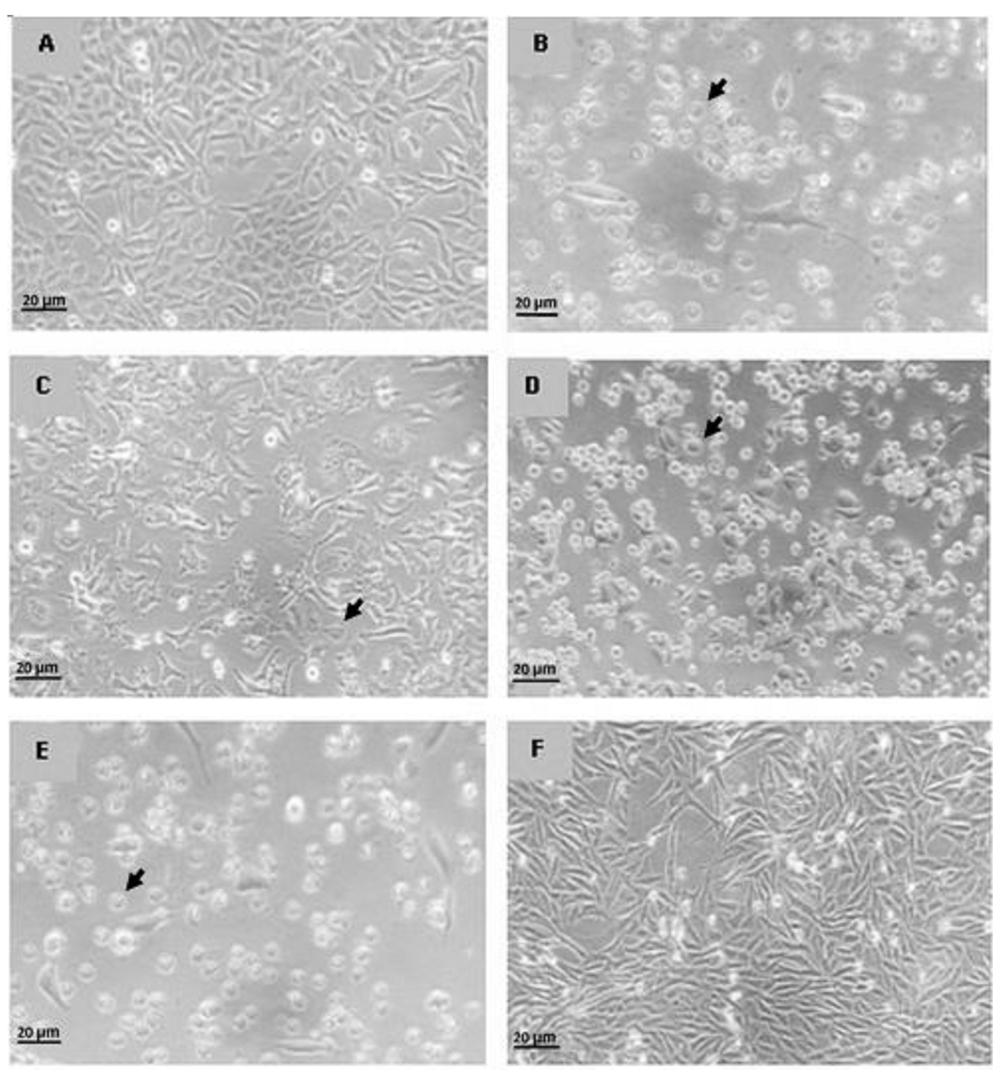
Control (A), Methanol (B), Dichloromethane:Methanol (C), Dichloromethane (D), Doxorubicin (E) and DMSO (F). Observation of cell morphology was performed using inverted microscope Axio Vert.A1 Zeiss with a magnification of 40x. Arrow shows dead cells.
Detection of apoptosis marker after treatment by extracts can be seen in Figure 3.
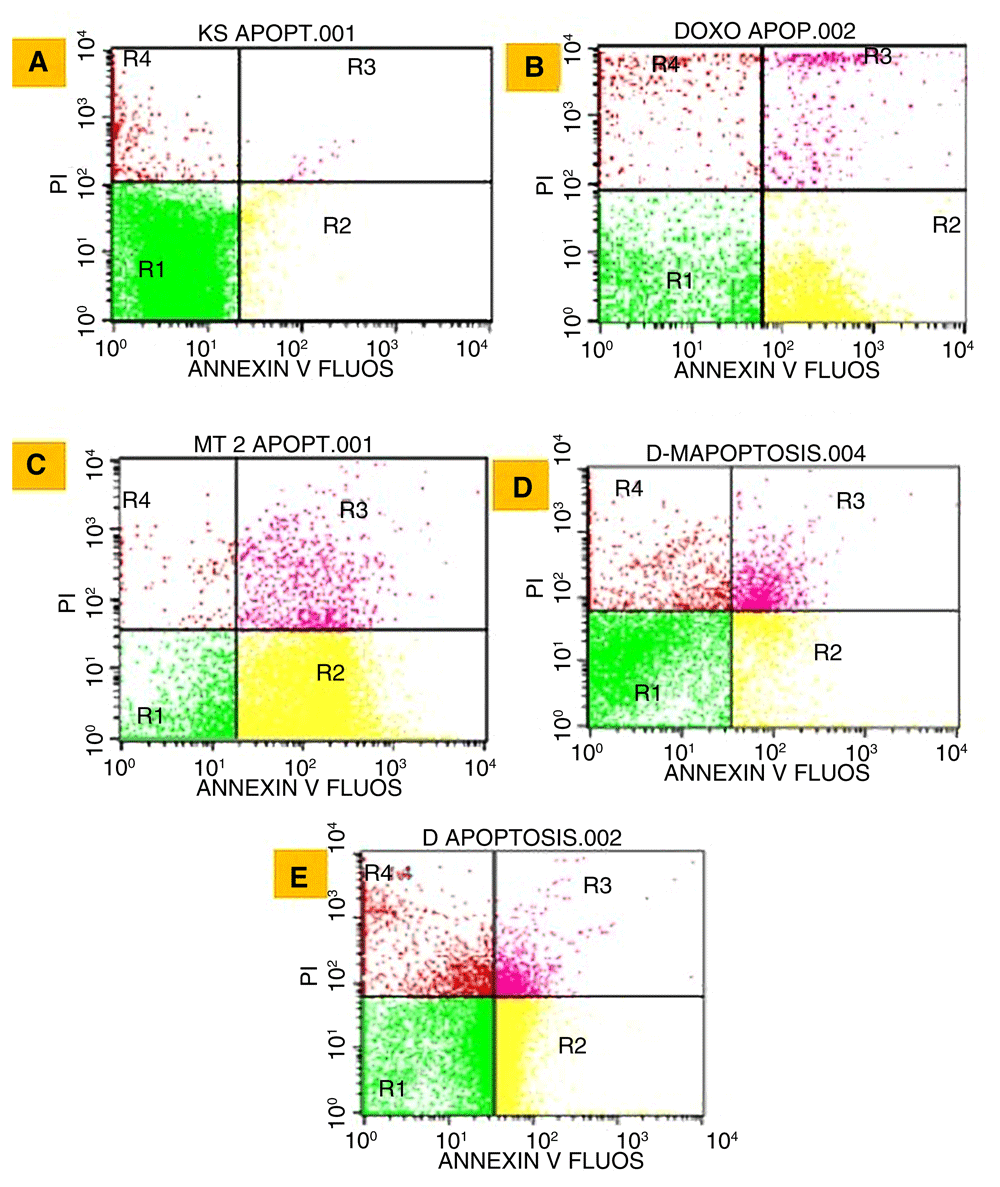
Cell control (A), doxorubicin (B), methanol (C), dichloromethane:methanol (1:1) (D), and dichloromethane (E). R1 (normal cells), R2 (early apoptosis cells), R3 (late apoptotic cells), and R4 (necrosis cells).
All Ancorina sp. extracts increase the percentage of apoptotic cells compared to control cells (Figure 3). The highest percentage of apoptosis was obtained in the methanol group (88.68%), which was even higher than doxorubicin as a positive control (75.74%) (Table 2).
The three extracts showed the same pattern with doxorubicin, i.e. a high percentage of apoptotic cell while the percentage of necrotic cells is low (Table 2).
We further investigated the apoptotic mechanism by examining the percentage of caspase-3. Detection of caspase-3 can be seen in Figure 4, while percentage of caspase-3 activation and correlation between percentage of apoptosis and caspase-3 activation can be seen in Table 3 and Figure 5, respectively.
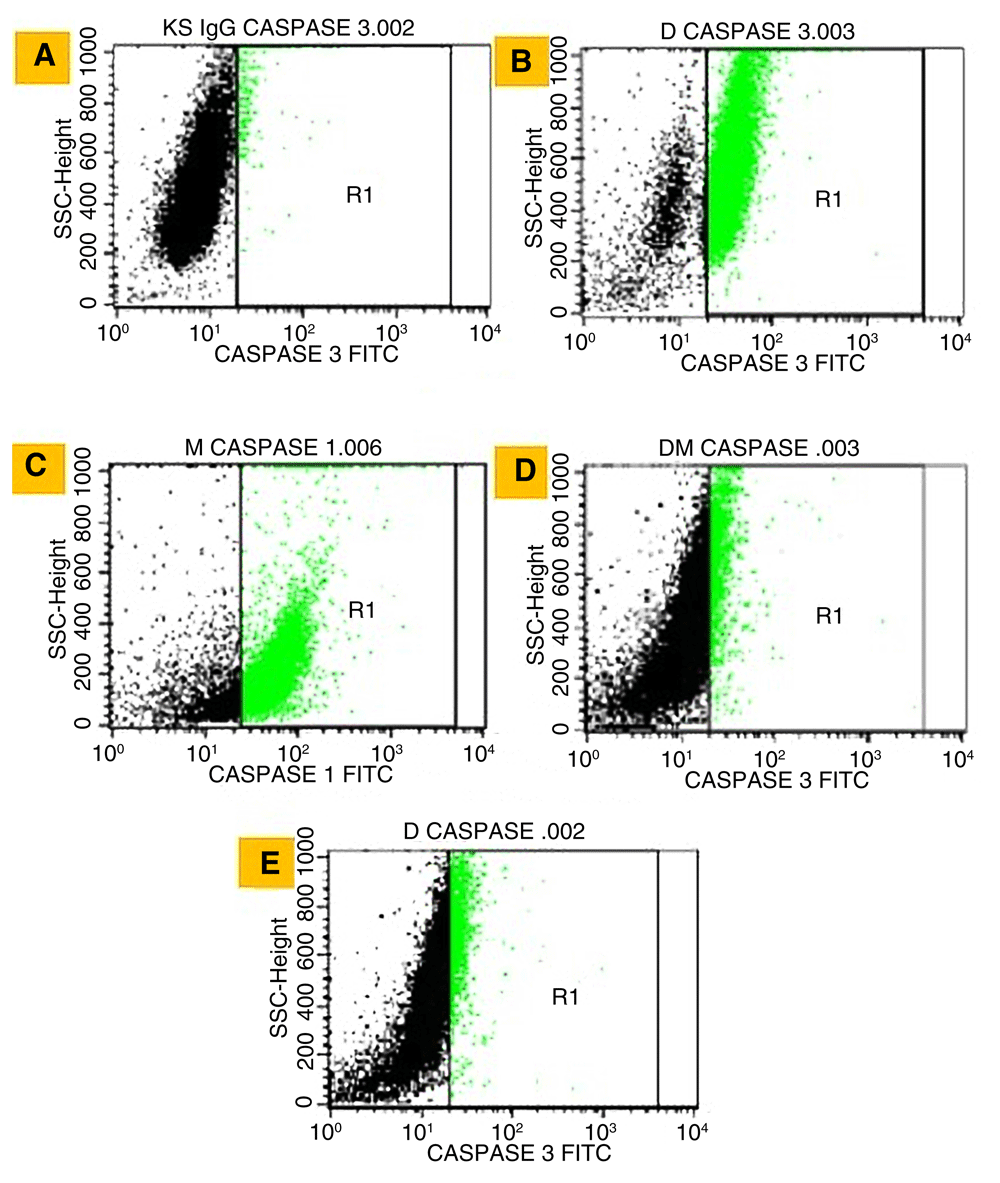
Negative control (A), Doxorubicin (B), Methanol (C), Dichloromethane:Methanol (D) and Dichloromethane (E). Black area indicated control cells while R1 showed caspase-3 activated cells.

Doxorubicin (Dox), Methanol (M), Dichloromethane:Methanol (1:1) (D:M), Dichloromethane (D), and negative control (control). Value after ± and error bar show standard deviation of two replications.
The highest percentage of caspase-3 was detected with methanol extract, which almost equaled doxorubicin, while the value of the other extracts was lower (Table 3).
The three extracts have a positive correlation between percentage of apoptosis and caspase-3. Although dichloromethane showed lower percentage of apoptosis and caspase-3, but they still have strong cytotoxicity (99.85 µg/mL), which shows potency as natural anticancer agents.
Sponges are highly diverse in Indonesia. In particular, encrusting sponges abundantly live in Gunung Kidul, Yogyakarta. Marine sponges produce some secondary metabolites, which can be used as antiviral22, antimicrobial22,23, antifungal22, and anticancer drugs17,24,29. The cell adhesion and immune system in sponge allow the different forms of the body plan30. When encrusting sponges grow together, sponges can survive by producing chemicals to kill fast dividing cells from the neighboring sponges. This ability of the chemicals can be used for chemotherapy since the basis of chemotherapy treatments is to disturb cancer cell growth31.
Sponge Ancorina sp. is a member of family Ancorinidae, which contains bioactive compounds such as ancorinoside BD, penazetidine A (Penares sollasi), ecionines A & B (Ecionemia sp.) and Iso malabaricane triterpenes (Rhabdastrella globostellata)32. Ancorinoside is a MT1-matrix metalloproteinase inhibitor in the development and metastasis of tumor cells33, whereas Penazetidine A strongly inhibits PKC-β1 activity in tumor cells with IC50 value 0.3 μg / mL34.
Ecionines A (biemnadin) and B (meridine) are anticancer compounds for many cancer cells, including bladder cancer cells33. Further, Iso malabaricane triterpenes were also found to be anticancer after testing on three types of cancer cells, namely L5178Y (mouse lymphoma), HeLa (human cervical carcinoma), and PC-12 (pheochromocytoma in mice)35. Ancorina sp. is a source of bioactive compounds such as ancorinoside A Mg salt, ancorinolates AC, bis-ancorinolate B, ancorinazole, indolo [3,2-a] carbazole, and (+) - 7-bromotrypargine36–38. These previous data show the high potency of Ancorinidae to be used as natural anticancer agents; hence this study is focused on the potency of Ancorina sp. as an anticancer agent and its mechanism, which is possibly through apoptosis induction.
Cytotoxicity is categorized into three levels by IC50 extract values. Very strong cytotoxicity has IC50 less than 10 μg / mL, strong cytotoxicity has IC50 values between 10 –100 μg/mL, and moderate cytotoxicity has IC50 values between 100 – 500 μg/mL39. According to these ranges, IC50 of the methanol and dichloromethane extracts in the present study had strong cytotoxic ability, while dichloromethane:methanol (1:1) extract belonged to moderate cytotoxicity. Ancorina sp. extracts have greater value of IC50 compared with the study34, which mentioned penazetidine A, a bioactive compound of marine sponge and highly inhibits PKC-β1 activity in tumor cells with lower IC50 of 0.3 μg/mL. This difference is due to the non-fractionated extract of our sponge, so that unsorted bioactive compounds possibly affect the cytotoxicity ability of extracts34.
Bioactive compounds from natural products depend on solvents. Based on the polarity of solvents, in the present study, Ancorina sp. extracts with polar solvent (methanol) lead to a higher apoptosis than non-polar (dichloromethane) or combination. These results are supported by a previous study that showed some compounds of Ancorinidae, such as ancorinoside BD, penazetidine A, echionines A and B and isomalabaricane triterpenes, are polar compounds that have anti-tumor and anti-cancer characteristics32. Interestingly, some studies in sponge also show same phenomenon such as cytotoxic compounds from sponge Dactylospongia elegans and Pachychalina alcaloidifera has been isolated using polar solvent methanol14,15. Terpenoids from sponge Iricina sp. and Spongia sp. have been isolated using polar solvent ethanol14,15,17. Bioactive compounds of sponge, both antiviral, antimicrobial, antifungal, and anticancer compounds have been successfully isolated by polar solvent as methanol19–21 and ethanol22. Considering all extracts in this study have low necrosis values (Table 2), they are safe to be used as medicine. Therefore, further studies are needed to find out the specific compounds of Ancorina sp. extracts.
Apoptosis can be triggered by extrinsic stimulation through death receptors on cell surfaces, such as TNFα (Tumor Necrosis Factor-α), Fas receptor (CD95 / APO1) and TRAIL (TNF related to ligand-inducing apoptosis) or by intrinsic stimulation through mitochondrial signaling pathways. In these two main pathways, activation of cysteine aspartyl proteases or caspase can produce mitochondrial permeabilization membrane, chromatin condensation and DNA fragmentation. These events stimulate the cells that are undergo apoptosis and lead to a distinctive cell morphology, such as the appearance of pyknosis, chromatin condensation, nucleus fragmentation, and apoptotic body formation, but organelles are still intact40. This can be seen in the present study in Figure 2.
Apoptotic pathways commonly occur by the activation of caspase-3, which is the effector of intrinsic, extrinsic and perforin pathways41. Caspase-3 is a key protease that is activated during the early stages of apoptosis. Caspase-3 is proteolytically active, cuts and activates other caspases, as well as relevant targets such as targets in the cytoplasm (D4-GDI and Bcl-23) and nucleus (poly (ADP-ribose) polymerase; PARP1)42.
In the present study, the highest percentage of caspase-3 was detected in methanol extract, which almost equal to doxorubicin, while the other extracts was lower (Table 3). Doxorubicin as a commercial drug in chemotherapy revealed a high percentage of apoptotic cells and caspase-3 activation. Among Ancorina sp. treatment groups, methanolic extract showed the highest percentage of both apoptosis and caspase-3. Interestingly, the methanolic extract showed a higher percentage than doxorubicin, and revealed its great potency to be used as a cancer medicine (Table 3).
The three extracts in this study have a positive trend between percentage of apoptosis and caspase-3 activation. Although dichloromethane showed a lower percentage of apoptosis and caspase-3, they had strong cytotoxicity (99.85 µg/mL) which shows potential as natural anticancer agents. It is possible that anticancer mechanism of dichloromethane and mixture of dichloromethane and methanol (1:1) excludes caspase-3 activation as effector caspases. Another pathway, such as caspase-6 or 7, can also induce apoptosis in T47D breast cancer cells27. More investigation is needed to elucidate the anticancer mechanism of these extracts.
All extracts of Ancorina sp. have strong or moderate cytotoxicity and have the ability to induce apoptosis in T47D human breast cancer cell line.
Open Science Framework: Apoptosis induction on human breast cancer T47D cell line by extracts of Ancorina sp., https://doi.org/10.17605/OSF.IO/AEJ9643
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This work was financially supported by Penelitian Dasar Unggulan Perguruan Tinggi No: 24/UN1/DITLIT/DIT-LIT/LT/2018 from Indonesia Ministry of Research, Technology and Higher education (to W.A.S.T).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We acknowledge Nur Faizah, Rudi Nirwantoro, and Ratih Aryasari for the fruitful discussions and helping in sponge sampling and identification.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell physiology, Cell signaling
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell physiology, Cell signaling
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Herbal medicine, complexity and Nano Biological approaches
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 10 Apr 19 |
read | read |
|
Version 1 07 Feb 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)