Keywords
Tooth bleaching, Hydrogen peroxide, hyper-sensitivity, Randomized clinical trial, in-office bleaching, VAS, desensitizing agent, ACP
This article is included in the All trials matter collection.
Tooth bleaching, Hydrogen peroxide, hyper-sensitivity, Randomized clinical trial, in-office bleaching, VAS, desensitizing agent, ACP
In-office bleaching permits close professional control, prevents material ingestion, reduces the total treatment time, and has the potential for quick results, improving patient satisfaction1. Be that as it may, tooth sensitivity (TS) is the most common side-effect of dental bleaching. It has been hypothesized that tooth sensitivity resulting from tooth bleaching occurs because peroxide penetrates the tooth structure and directly activates a neuronal receptor and not because of hydrodynamic effects. In addition, alteration caused by bleaching agents on the morphological enamel surface as increased surface porosity, depressions and superficial irregularities2,3.
For this reason, a desensitizing agent is used to decrease the tooth sensitivity that patients experience. The mechanism of action of potassium nitrate remains unknown. This acts by diminishing the excitability of the intra-dental nerve endings and blocking the dentinal tubules4. Potassium nitrate and sodium fluoride are broadly used as desensitizing agents to treat tooth sensitivity. Potassium ions cause the depolarization of the sensory nerve5, and fluoride treats tooth sensitivity, probably by blocking uncovered dentinal tubules or decreasing the liquid stream into the mash and blocking the transmission of improvements6. Of late, bioactive materials such as ACP (amorphous calcium phosphate) and CCP-ACP (casein phosphopeptite and amorphous calcium phosphate) as desensitizers and re-mineralizing specialists have been utilized for this purpose; however, there is only limited research in this area7. The objective of this randomized clinical trial is to assess whether the use of a desensitizing agent will diminish the tooth sensitivity induced by dental bleaching.
The protocol and the informed consent template were reviewed and accepted by the Ethics committee of Scientific Research at the Faculty of Dentistry, Cairo University on October 2016 (Approval number 161038 on 24 October 2016). All participants provided written informed consent regarding all of the trial’s procedural steps and the publication of the trial results and photos. The trial has been registered on clinical-trials.gov (NCT02942082) on 21 October 2016.
This was a randomized clinical trial with an equal allocation ratio (1:1:1:1). This article was prepared following the protocol established by the consolidation standards of reporting trials, statement 2010. The trial was started in November 2017 and it was completed in November 2018.
All participants were recruited from the outpatient clinic of the Conservative Department at Cairo University who attended seeking tooth whitening. In total, 36 (6 male and 30 female) adult patients took part in this trial subsequent to satisfying all inclusion criteria. Telephone numbers and addresses of all subjects in the study were recorded as part of the signed consent.
All subjects received a phone call at the time of the pre-determined follow-up dates. Patients were given a brief explanation about the investigations those who agreed to participate signed an informed consent. The in-office bleaching was performed in a single visit.
To be included in this trial, participants needed to be aged between 18 and 40 years old, have good oral health with no periodontal disease and have six upper anterior teeth that are free from either caries or restoration with no sign of spontaneous pain.
Participants with anterior restorations and carious lesions in their upper labial surfaces were not included in this trial. Additionally, participants suffering from recession and cracks, those taking analgesics, and those with bruxism habits were also not enrolled in this trial. Finally, pregnant women and lactating mothers were also excluded.
Sample size calculation was performed using the G*Power program version 3.1.9.2 (University of Düsseldorf, Düsseldorf, Germany). Based on post-bleaching hypersensitivity results from previous studies8,9, a sample size of 36 patients was calculated. A sample of 24 patients was enough for the detection of an effect size 0.4, with a power of 85%, and a 5% significance level. The number was increased to 29 to address non-parametric variables in order to increase the reliability of the results, and again increased to 36 for loss to follow up.
Using a free online website (http://www.random.org/), a sequence generation for patient numbers was created. Each participant chose a number from successively numbered obscure envelopes. They were then allocated into one of the set-ups using a randomization table formed of four columns, and each column contained numbers from one to nine. The table was kept with the senior supervisor (MA) who generated the allocation sequence and he was responsible for ensuring proper randomization and allocation concealment.
After taking a medical and dental history using medical and dental health charts see (extended data10), patients were given a concise clarification regarding the examinations. A prophylaxis paste and a low-speed brush for teeth cleaning were used a day prior to bleaching. Utilizing a visual analogue scale (VAS, see extended data10), every member set a mark that represents their level of pain on a scale from 0–10. VAS scores were assessed for each patient by measuring the distance in cm from the anchor word (0 cm) to the mark.
In-office bleaching was done using Dash™ whitening gel syringe 30% hydrogen peroxide (Discus dental, Culver City, 91761 USA) batch number DSE 1001, for three sessions each 15 minutes.
In-office bleaching procedures. After patient lip lubrication, the operator placed a cheek retractor and a bit block into the patients mouth (Figure 1). Isolation using cotton rolls was performed, and a liquid barrier material, Liquidam™ barrier syringe catalogue number 99-905-26, was applied to cover the tissues. The applied Liquidam was cured for 10 seconds. Then, according to the group specification, the following were performed: Group I: a desensitizing agent Relief® ACP oral care gel syringe (Discus Dental, Culver City, 91761 USA) batch number DSE 1001 which according to the manufacture, contains 5% potassium nitrate; 0.22% sodium fluoride and 0.75% amorphous calcium phosphate (ACP) was applied on the teeth before dental bleaching; Group II: the Relief® ACP was applied before and after bleaching procedures; Group III: Relief ACP was applied after bleaching; and Group IV: glycerin liquid (Human Care International, Egypt, Batch No. SP8P) was applied before and after bleaching procedures. Dash™ whitening gel syringe 30% hydrogen peroxide (Discus dental, Culver City, 91761 USA) batch number DSE 1001 was applied to the facial side of teeth (1–2 mm thick), and the gel was left on the teeth for 15 minutes for a total of three cycles (Figure 2). After each cycle, the gel was removed with surgical suction tip or wiped with gauze. Again, the desensitizing agent Relief® ACP Oral Care Gel or glycerin was applied on the labial surfaces of teeth according to the group specification. For Group II, Group III, and Group IV, glycerin was applied before and after in-office bleaching. Post-operative instructions were given to the participants; for the next two days, they were asked to avoid pigmented consumption, such as tea and cola, and to avoid using toothpaste with desensitizing agents.
Post-bleaching hypersensitivity. Post-bleaching hypersensitivity was assessed using a VAS scale, immediately and 24 hours, one week and one month after the bleaching procedure. VAS was used by the patients from the second day after bleaching on a daily basis to record tooth sensitivity between the schedule of the subsequent evaluations, and patients were asked to bring this pain diary at every point of assessment. The assistant supervisor (ME) kept in contact with the participants by phone to remind them and to ensure accurate adherence to instructions. VAS scores were assessed for each patient by measuring the distance in cm from the anchor word (0 cm) to the mark. The blinded assessor collected the data of evaluation in a printed assessor chart (extended data10).
Color evaluation. The blinded assessor recorded the shade of each participant’s teeth at baseline and at the recall appointments (after 24 hours, 1 week and 6 months). The measurement area of interest for shade matching was the middle third of the facial surface of maxillary anterior teeth. 16 tabs of the shade guide (The VITAPAN ® classical shade guide (Vita Zahnfabrik, Badsä ckingen, Germany) were arranged from highest (B1) to lowest (C4) value. These values were converted into numerical codes using a conversion table (Table 1) (Borges et al., 2012).
This was a double-blind clinical trial in which the participants and assessors were unaware of the type of the desensitizer used (relief® ACP oral care gel or a placebo) and were not aware of which group they were in. However, for clinical purposes, the operator was not blind, as both materials used had the same color but different consistencies; thus, it was impossible to blind the operator. The assessor collected the data from evaluations in a printed assessor chart (see extended data10).
In this study, data analysis was performed using IBM SPSS statistical for Windows, Version 23.0 Armonk, NY:IBM Corp. Non-normal (non-parametric) data were represented as the median and Inter-quartile Ranges (IQR). For comparison between the four groups, a Kruskal–Wallis test was used. Friedman’s test was used to study changes over time within each group. For pairwise comparison, Dunn’s test and Bonferroni’s test were used. Quantitative data were represented in terms of frequencies and percentages, and significance was set at P ≤ 0.05.
In this study, 36 (6 male and 30 female) adult patients took part in this trial (see underlying data). A patient flow chart is available as part of the Reporting guidelines10.
Patient ages ranged between 18–40 years old; their mean ages were 32.4± 4.3, 32.1 ± 4.4, 33.1 ± 4.8 and 32.1± 5.3 for the ACP gel before bleaching group, ACP gel before and after bleaching group, ACP gel after bleaching and the control group, respectively, with no statistically significant difference regarding mean age values (P-value = 0.965) between all groups.
Regarding patient gender, the ACP gel before bleaching group included two males (22.2%) and seven females (77.8%), while the ACP gel before and after bleaching group included one male (11.1%) and eight females (88.9%), the ACP gel after bleaching group included on male (11.1%) and eight females (88.9%) and the control group, glycerin before and after bleaching, included two males (22.2%) and seven females (77.8%). Regarding gender distribution, no significant difference (p = 1.000) was found. (Table 2)
A comparison of the VAS scores is shown in Table 3 and Figure 3. Before bleaching, all cases in the study groups showed no pain (P-value = 1.000, Effect size = 0.000). A significant difference was shown in all groups immediately after bleaching (P-value <0.001, Effect size = 3.149). Regarding pairwise comparison between the groups, the ACP gel after bleaching and glycerin before and after bleaching groups showed the highest median pain score of 5 (4–6.5) and 5 (5–7), respectively, with a non-statistically significant difference between both groups and that of the ACP gel before bleaching group, which was 4 (3.5–4.5). The ACP gel before and after bleaching group showed the lowest median pain score of 3 (0–4), with a non-statistically significant difference from the ACP gel before bleaching group, which was 5 (5–7), and a statistically significantly lower median pain score than the ACP gel after bleaching and glycerin applied before and after bleaching groups, at 5 (4–6.5) and 5 (5–7), respectively. After 1 day, a significant difference was revealed between the groups (P-value < 0.001, Effect size = 3.367). Pairwise comparison between the groups revealed that the glycerin before and after bleaching group showed the highest median pain score of 3 (2–3.5), with a non-statistically significant difference from the ACP gel after bleaching group, which was 2 (1–2.5). The ACP gel before and after bleaching group showed the lowest median pain score 0 (0–1), with a non-statistically significant difference from the ACP gel before bleaching group, at 1 (0.5–1.5), and a statistically significantly lower median pain score than the ACP gel after bleaching and glycerin applied before and after groups, at 2 (1–2.5) and 3 (2–3.5), respectively. On day 2 after bleaching, a statistically significant difference was shown (P-value = 0.099, Effect size = 1.046). For days 3, 4, 5, 6 and 7, and even after 1 month, all cases of the four groups showed no pain.
| Time | ACP gel before bleaching (n = 9) | ACP gel before & after bleaching (n = 9) | ACP gel after bleaching (n = 9) | Glycerin before & after bleaching (n = 9) | P-value | Effect size (Eta Squared) |
|---|---|---|---|---|---|---|
| Before bleaching | 0 (0 – 0) | 0 (0 – 0) | 0 (0 – 0) | 0 (0 – 0) | 1.000 | 0.000 |
| Immediately after bleaching | 4 (3.5 – 4.5) AB | 3 (0 – 4) B | 5 (4 – 6.5) A | 5 (5 – 7) A | <0.001* | 3.149 |
| Day 1 | 1 (0.5 – 1.5) B | 0 (0 – 1) B | 2 (1 – 2.5) A | 3 (2 – 3.5) A | <0.001* | 3.367 |
| Day 2 | 0 (0 – 0.5) | 0 (0 – 0) | 0 (0 – 0) | 0 (0 – 1) | 0.099 | 1.046 |
| Day 3 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 1.000 | 0.000 |
| Day 4 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0 – 0) | 1.000 | 0.000 |
| Day 5 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0 – 0) | 1.000 | 0.000 |
| Day 6 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0 – 0) | 1.000 | 0.000 |
| Day 7 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0 – 0) | 1.000 | 0.000 |
| 1 Month | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 1.000 | 0.000 |
Results for changes in hypersensitivity are presented in Table 4 and Figure 4. In the ACP gel before bleaching group, the median pain score values changed statistically significantly over time (P-value < 0.001, Effect size = 0.437). The pair-wise comparison showed a statistically significant rise in the 0 median pain results instantly after bleaching followed by a statistically significant reduction in pain results after 1 day. Days 1 to 2 did not alter pain statistically significantly. No statistically significant change was observed in the pain scores after bleaching from day 2 to days 3, 4, 5, 6, 7 and 1 month.
Similarly, there was no statistically significant variation in pain score in the ACP gel before and after bleaching group after bleaching over time (P-value of < 0.001, Effect size of 0. 484). A comparison of the time points shows that the mean pain scores instantly after bleaching increased statistically significantly followed by a statistically significant reduction in pain results after the first day. Days 1 to 2 did not alter the pain results statistically significantly. No statistically significant changes to pain values after bleaching occurred from day 2 to days 3, 4, 5, 6, 7 and 1 month, as all the cases did not have any pain.
| Time | ACP gel before bleaching (n = 9) | ACP gel before & after bleaching (n = 9) | ACP gel after bleaching (n = 9) | Glycerin before & after bleaching (n = 9) |
|---|---|---|---|---|
| Before bleaching | 0 (0 – 0) B | 0 (0 – 0) B | 0 (0 – 0) C | 0 (0 – 0) C |
| Immediately Post bleaching | 4 (3.5 – 4.5) A | 3 (0 – 4) A | 5 (4 – 6.5) A | 5 (5 – 7) A |
| 1 Day | 1 (0.5 – 1.5) B | 0 (0 – 1) B | 2 (1 – 2.5) B | 3 (2 – 3.5) B |
| 2 Days | 0 (0 – 0.5) B | 0 (0 – 0) B | 0 (0-0)C | 0 (0 – 1)C |
| 3 Days | 0 (0-0) B | 0 (0-0) B | 0 (0-0)C | 0 (0-0)C |
| 4 Days | 0 (0-0) B | 0 (0-0) B | 0 (0-0)C | 0 (0-0)C |
| 5 Days | 0 (0-0) B | 0 (0-0) B | 0 (0-0)C | 0 (0-0)C |
| 6 Days | 0 (0-0) B | 0 (0-0) B | 0 (0-0)C | 0 (0-0)C |
| 7 Days | 0 (0-0) B | 0 (0-0) B | 0 (0-0)C | 0 (0-0)C |
| 1 Month | 0 (0-0) B | 0 (0-0) B | 0 (0-0)C | 0 (0-0)C |
| P-value | <0.001* | <0.001* | <0.001* | <0.001* |
| Effect size (W) | 0.437 | 0.484 | 0.298 | 0.466 |
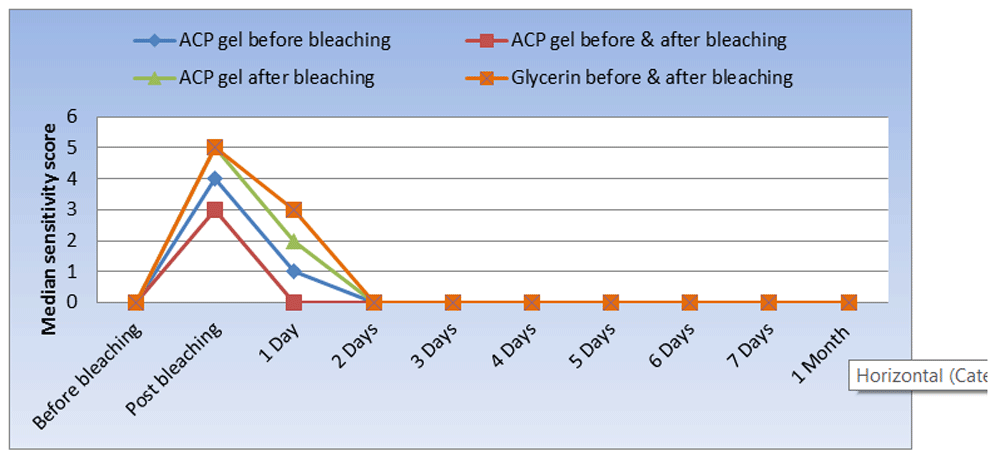
With regard to the ACP gel after bleaching group, the median pain scores over time (P-value < 0.001, Effect size= 0.298) changed significantly. The comparison between time intervals indicates that median pain scores were statistically significantly increased instantly after bleaching, and that the pain scores decreased statistically significantly after 1 day and from 1 to 2 days. Between day 1 and day 2, there was no statistically significant shift in pain rates.
No statistically significant changes in pain scores after bleaching occurred between day 2 and days 3, 4, 5, 6, 7 and 1 month, as there was no pain in all cases.
In the same way, the median pain results changed over time (P-value < 0.001, Effect size = 0.466) in the glycerin before and after bleaching group. A pairwise comparison between times showed that the median pain scores results increased statistically significantly immediately after the bleaching and then decreased after 1 day and from 1 day to 2 days. No significant changes in pain scores were observed from 2 days to 3 days.
Comparison between interventions. Results of changes in tooth shade across groups are presented in Table 5 and Figure 5. There was no statistically significant difference between the groups immediately post bleaching, after 1 day and 1 week (P-value = 0.828, Effect size = 0.066), 1 month (P-value = 0.426, Effect size = 0.007), or 6 months (P-value = 0.024, Effect size = 0.202). Pair-wise comparison between the groups revealed that the ACP gel before & after bleaching group showed the statistically significantly highest median change in classical shade guide score. ACP gel after bleaching group showed a lower median change in shade, followed by the ACP gel before bleaching group which where statistical significant. Glycerin before & after bleaching group showed the lowest median change in shade guide score.
| Time | ACP gel before bleaching (n = 9) | ACP gel before & after bleaching (n = 9) | ACP gel after bleaching (n = 9) | Glycerin before & after bleaching (n = 9) | P-value | Effect size (Eta Squared) |
|---|---|---|---|---|---|---|
| Post bleaching | -8 (-11 – -6.5) | -11 (-11.5 – -7.5) | -9 (-12.5 – -6.5) | -10 (-12 – -6.5) | 0.828 | 0.066 |
| 1 Day | -8 (-11 – -6.5) | -11 (-11.5 – -7.5) | -9 (-12.5 – -6.5) | -10 (-12 – -6.5) | 0.828 | 0.066 |
| 1 Week | -8 (-11 – -6.5) | -11 (-11.5 – -7.5) | -9 (-12.5 – -6.5) | -10 (-12 – -6.5) | 0.828 | 0.066 |
| 1 Month | -8 (-10.5 – -6) | -11 (-11.5 – -7.5) | -9 (-11.5 – -6.5) | -7 (-11 – -6) | 0.426 | 0.007 |
| 6 Months | -7 (-10.5 – -5) C | -11 (-12.5 – -7) A | -8 (-10 – -6) B | -4 (-7.5 – -1.5) D | 0.024* | 0.202 |
The in-office bleaching method offers great efficiency, using hydrogen peroxide with elevated concentrations of 25 to 40%3. High-concentration bleaching agents, however, cause tooth sensitivity, which is the primary adverse impact associated with in-office bleaching, during and up to 24 hours following the bleaching operation. This sensitivity is associated with the pulp tissue inflammation phase11,12.
In each clinical session, 30% hydrogen peroxide in-office bleaching was performed according to the guidelines of the manufacturer for three 15-minute sessions in this research. Several studies have implemented hydrogen peroxide in three 15-minute sessions13–16. H2O2 produces free radicals, which interact with pigment molecules to have a whitening impact. These free radicals in the bleaching gel break down the double bonds between pigment molecules and change the arrangement and/or size of the pigment molecules, creating a more white tooth appearance17.
The release of cell-derived factors, such as ATP and prostaglandins, can be caused by tooth bleaching. This interaction can cause pulp nociceptors to be excited or sensitized, and harm the pulp tissue18. Another hypothesis is presently not well recognized; i.e., that the hydrodynamics activate the intradental nerve and release neuropeptide in reaction to this operation3.
Potassium nitrate and sodium fluoride are often used to treat tooth sensitivity as desensitizing agents. These agents can be contained in a bleaching gel and supplied during therapy with a custom tray, or applied separately by being placed in the mouth of the subject for a brief period of time prior to bleaching19,20. In contrast, fluoride used as a desensitizing agents treats tooth sensitivity by blocking exposed dentinal tubules or decreasing liquid pulp flow and blocking stimulus transmission6.
The mechanism by which potassium nitrate operates is still not known. Various randomized clinical studies to assess the effectiveness of tooth bleaching have been released, reporting that the use of potassium nitrate and sodium fluoride eliminate post bleaching hypersensitivity14,16,18,20–23. A systemic review and meta-analysis assessed the effectiveness of potassium nitrate and sodium fluoride as desensitizing agents in tooth bleaching therapy and concluded that potassium nitrate and +/-sodium fluoride decrease tooth sensitivity24.
The supply of Ca and PO4 ions, by means of ACP technology, together with whitening processes can be useful to minimize the loss of minerals as well as the occurrence of roughness and erosion in the dental structure due to whitening25. There is limited research on the application of ACP, bioactive materials, CCP-ACP, bio-glass and bio-glass ceramics as desensitizing agents and re-mineralizing agents7.
The primary aim of these products is to encourage dental structural remineralization through the creation of an amorphous calcium phosphate layer on the surface of the product, initiating apathetic crystallization. This method of remineralization seems far more efficient than sodium nitrate or fluoride alone26, as it not only helps to alleviate the pain, but also stops it from beginning. (Zhao et al. 2011)27 They reviewed the application of amorphous calcium phosphate in dentistry and found that, as a result of increasing microcrystalline, ACP is converted readily into a crystalline phase, such as octacalcium phosphate and apatite.
A study by Kwon et al., 201628 evaluated the time taken by potassium nitrate to reach the pulp cavity and the impact of penetration concentrations on tooth whitening. Relief ACP was used for 0, 5, 15, 30 and 60 minutes before bleaching. The study concluded that, as early as 5 minutes after application, potassium nitrate penetrates into the pulp cavity, and potassium nitrate pretreatment with desensitizers had no adverse effect on the efficacy of tooth whitening. Similar penetration times for hydrogen peroxide and the penetration of potassium nitrate can be described by the small molecular weights of both 34.40 grams per mol and 101,10 grams per mol, respectively; and also, as water soluble agents, their transport into the tooth can be facilitated28. The present clinical trial used oral Relief ACP® gel as a desensitizing agent that, according to the manufacturer, includes 5% potassium nitrate, 0.22% sodium fluoride and 0.75% amorphous calcium phosphate (ACP)4.
In the present trial, a VAS scale was used, as a great deal of proof is available to support the pain intensity validity of VAS. Such scales show benefits over other pain intensity self-reporting measures and pain-controlled behavior29,30. Many studies have used the VAS scale to assess dental sensitivity intensity after office bleaching8,9,14,31–34. Others have used both the visual analog scale (VAS) and numerical scale (NRS)16,23.
In this study, participant ages in all groups ranged from 18–40 years old. The mean of ages of patients were 32.4± 4.3, 32.1 ± 4.4, 33.1 ± 4.8 and 32.1± 5.3 for the ACP gel before bleaching group, ACP gel before and after bleaching group, ACP gel after bleaching group and control group, respectively, with no statistically significant difference regarding mean age values (P-value = 0.965) between all groups. A recent review was conducted in 35 (Rezende et al., 2016). Regarding the participants’ ages, the authors did not find any link between age and the risk or level of tooth sensitivity. Regarding gender, 36 patients (30 women and six men) took part in this research. There was no statistical significance in gender distribution among all groups.
However, with the incidence of female patients in this research distinct outcomes for other population profiles can be noted. Furthermore, socio-cultural beliefs about femininity and men also seem to be an important factor in pain response between the sexes, as the social acceptance of pain expression among females is usually more acceptable—an influence that could lead to biased pain reporting36. The highest average pain score was found in the ACP gel after bleaching and Glycerin before and after bleaching groups, showing the highest median pain score. The lowest average pain score was shown in the ACP gel applied before and after bleaching group. As mentioned above, potassium nitrate has advantages as a product in that it reduces tooth sensitivity after dental bleaching; several clinical studies have reported this14,16,18,20–23. Moreover, the efficacy of potassium nitrate and sodium fluoride as desensitizing agents was measured in a systematic review meta-analysis24. These studies cannot be directly compared with this clinical trial, as the Relief ACP ® oral care used as a desensitizing agent includes in its ingredients 5% potassium nitrate, 0.22% sodium fluoride and 0.75% amorphous calcium phosphate (ACP).
In this research, hypersensitivity for all participants tested was not reported by the end of the seven days of the trial. This conclusion was consistent with that in Martin et al., and Farag et al.8,37.
In a control group in which glycerin was used as a placebo before and after in-office bleaching, a hypersensitivity enhancement was noted. This may be the result of the placebo or Hawthorne effect. The Hawthorne effect can be defined as an unconscious shift in the participant because of the simple understanding of being observed during a clinical trial38.
This trial was a randomized clinical trial with a comparatively large sample of patients, performed in a clinical setting. This was the first clinical study to use a desensitizing agent before and after in-office bleaching to reduce hypersensitivity after bleaching. The median age for patients in this study was 32, and this affects the generalizability of the results to the general population and can be regarded as a study limitation. The following limitation should be considered: the generalizability of results may be limited given the number of female participants was much higher than male participants. We assessed one bleaching product, Dash™ whitening gel; different bleaching gels and concentrations are likely to affect sensitivity. A randomized clinical trial should therefore be performed with various bleaching products and demographic profiles using other equivalent measures.
The application of the desensitizing agent before and after bleaching was found within the limitations of this research, to be effective in reducing post-bleaching hypersensitivity. Hypersensitivity in all groups gradually decrease two days after in-office bleaching.
Written approval was acquired from patients to publish their clinical information and clinical images.
Open Science Framework: Evaluation of post-bleaching hypersensitivity using desensitizing agent before and /or after in-office bleaching: A randomized clinical trial. https://doi.org/10.17605/OSF.IO/XUB2510
The project contains the following underlying data:
Open Science Framework: Evaluation of post-bleaching hypersensitivity using desensitizing agent before and /or after in-office bleaching: A randomized clinical trial. https://doi.org/10.17605/OSF.IO/XUB2510
This project contains the following extended data:
Supplementary files
Assessor chart (Word document containing chart used by assessor to record patient data)
Pain diary (Word document containing pain diary with VAS scales used by participants)
Medical and dental health charts (Word document containing chart used to record medical and dental data for participants on requirement)
Open Science Framework: CONCORT checklist and flow diagram for Evaluation of post-bleaching hypersensitivity using desensitizing agent before and /or after in-office bleaching: A randomized clinical trial. https://doi.org/10.17605/OSF.IO/XUB2510
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Restorative Dentistry
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Bersezio C, Estay J, Sáez M, Sánchez F, et al.: Six-month Follow-up of the Effect of Nonvital Bleaching on IL-1β and RANK-L: A Randomized Clinical Trial.Oper Dent. 44 (6): 581-588 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical studies, Dental materials, Tooth whitening
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Oct 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)