Keywords
Functional dyspepsia, postprandial distress syndrome, global overall symptom scale, Curcuma longa, simethicone
This article is included in the All trials matter collection.
Functional dyspepsia, postprandial distress syndrome, global overall symptom scale, Curcuma longa, simethicone
Dyspepsia is a common functional gastrointestinal disorder which affects 20% of the global population1. Although dyspepsia is not a life-threatening condition, it disrupts the quality of life and also socioeconomic impaction for suffering patients2–4. The United States population spends $18 billion annually on dyspepsia management4. In Thailand, the prevalence of dyspepsia is higher than global prevalence, affecting more than half of the Thai population5.
Rome IV criteria for diagnosis classifies functional dyspepsia (FD) into two groups based on symptoms; (1) postprandial distress syndrome (PDS), consisting of postprandial fullness and early satiety, and (2) epigastric pain syndrome (EPS)6. Currently, proton pump inhibitors (PPI) are regarded as an effective treatment for FD but ineffective in relieving PDS symptoms6. Therefore, physicians frequently consider prescribing other agents for these patients.
Pathogenesis of FD is likely complex and multifactorial. The factors that cause PDS comprise delayed gastric emptying time, impaired gastric accommodation, and gut inflammation6. In addition, psychosocial factors such as anxiety, depression and psychiatric disorders also induce pathogenesis6.
Simethicone is a defoaming agent. Foam, formed by gas in the gastrointestinal tract (GI) and gastric mucous, is a cause of fullness if it accumulates in GI tract7. Therefore, foam reduction can increase gastric emptying time and relieve postprandial fullness8–13. Many studies have found that simethicone has efficacy for treatment of dyspepsia and no serious adverse reaction14–17.
Curcuma longa is a Thai herb that effectively relieves flatulence18. Previous rodent studies19–21 documented that Curcuma longa can decrease gut inflammation via its active ingredient curcumin (R = OCH3, R' = OCH3). Curcumin inhibits many proinflammatory enzymes such as cyclooxygenase-2, 5-lipoxygenase, and inducible nitric oxide synthase enzymes etc. Not only does it have an anti-inflammatory effect, but curcumin also increases gastric emptying time and reduces depressive symptoms via the brain-gut axis22. Thus, Curcuma longa is commonly used for FD treatment.
Many human studies supported the efficacy of Curcuma longa compared with other agents for treatment of dyspepsia19,22,23.A trial in 2007 found that taking a 2-g Curcuma longa capsule daily for four weeks indicated no significant difference with ranitidine for dyspepsia relief23. A later study in 2016 showed that addition of curcumin on top of the standard anti-helicobacter regimen in patients with peptic ulcers was safe and improved symptoms of dyspepsia but did not enhance effect on the eradication of Helicobacter pylori infection24.
However, no current evidence of the efficacy of Curcuma longa as compared with simethicone for PDS symptoms. Thus, the aim of this present study was to assess the efficacy of Curcuma longa compared with simethicone in patients with PDS.
Adults (age 20–60 years) with FD, diagnosed during a routine clinical appointment by physicians working at any Social Medicine clinic of Khon Kaen Hospital (Khon Kaen, Thailand), on the basis of Rome IV criteria6, were screened by nurse officers for participation then enrolled in the study by the principal investigator. Inclusion criteria included postprandial distress syndrome, no alarm features, and discontinuation of all GI drugs at least one week before randomization. Patients with a history of either simethicone or Curcuma longa allergy, gastric malignancy, gallstone or biliary obstruction, pregnancy, and on breastfeeding period were excluded. Written informed consent was obtained from all patients.
The Khon Kaen Hospital Institute Review Board in human research approved the study protocol. This trial was registered with the Thai Clinical Trials Registry on 31st January 2018; registration number, TCTR20180131001. This randomized, active-comparator, open-label trial was conducted at primary care clusters of Khon Kaen Hospital, and Nam Pong Community Hospital between July 2018 and February 2019. All the authors were involved in the design and performance of the study, which was conducted according to the Declaration of Helsinki. First research assistant (CT) used computer-generated simple randomization and sequentially labeled the number on opaque drug containers for concealment. After the principle investigator (NS) enrolled participants, the second research assistant (MJ) then assigned the concealed interventions in order. There was no deviation from the original trial protocol.
Patients were randomly assigned to take 750 mg Curcuma longa capsule per day, 1500 mg of Curcuma longa capsule per day or 240 mg of simethicone per day for four weeks. All patients were educated on lifestyle modification, such as stopping drinking and smoking, decreasing spicy foods and the volume eaten per meal, and trying to choose foods with softer consistentcy. Female participants were given a urine pregnancy test, and were excluded if result indicated positive. Baseline characteristics were measured together with BMI and global overall symptom (GOS) scale (described below). Medication was administered orally 30 to 60 minutes after each meal (250 mg (one Curcuma longa capsule per meal), 250 mg (two Curcuma longa capsule per meal), or 80 mg (one simethicone tablet per meal) three times per day). Patient's visits were scheduled at the start of treatment and at the end of 2, 4, and 6 weeks (2 weeks after stopping treatment). All patients discontinued medication after week 4 and reported for recurrence of symptoms at week 6. Patients also completed daily logs of symptoms and adverse events during the week preceding each visit. At each visit, the patient, with the principle investigator (NS), completed the seven-point GOS scale for dyspepsia (which ranges from 1 to 7, with 1 indicating no problem, 2 indicating minimal problem (can be easily ignored without effort), 3 indicating mild problem (can be ignored with effort), 4 indicating moderate problem (cannot be ignored but does not influence my daily activities), 5 moderately severe problem (cannot be ignored and occasionally limits my daily activities), 6 indicating severe problem (cannot be ignored and often limits my concentration on daily activities), and 7 indicating very severe problem (cannot be ignored and markedly limits my daily activities and often requires rest)25.
At the last visit, patients were asked to report their recurrence of symptoms and the date of recurrence after discontinuation of treatment.
We focused on PDS symptoms, measured using the GOS scale, so we combined the early satiety score and postprandial score as the composite outcome. The two primary endpoints were the comparison of mean changes of composite outcome between groups from baseline to week 2 and week 4. Secondary end points were rates and durations of recurrences at week 6, and also adverse effectsas assessed by a daily log of adverse events. Post-hoc analyses included the changes from baseline in each group at week 2 and week 4.
We calculated that a sample of 69 patients would provide adequate power for the proposed tests in this three-group study using the formula for sample size calculation to compare k means by one-way ANOVA pairwise, 2-sided equality26. By substitution of mean in treatment group (µtrt) = -1.8624, mean in control group (µcon) = -1.3024, SD in each group = 0.6524, α = 0.05 and β = 0.20, r = 1, n = 22 per group was be derived.
SPSS version 24.0 was used. Mean changes from baseline to week 2 and week 4, in GOS scale, were analyzed with one-way ANOVA. Dichotomous endpoints (recurrent rates) were compared among the groups with the use of Chi-squared and Z-test. The duration of symptoms was compared using Kruskall-Wallis test. Means among the groups were also analyzed using one-way ANOVA. To compare means in each group (before and after), a paired t-test was used. Bonferroni post-hoc test was used for post-hoc analysis.
A total of 94 patients with functional dyspepsia were assessed for eligibility. There were 16 patients excluded from study due to not meeting inclusion criteria (n= 14) and declined to participate (n=2). A total of 78 patients underwent randomization. There were 27 in the 750 mg Curcuma longa group, 26 in 1500 mg Curcuma longa group and 25 in the simethicone group; there were 6, 4, and 2 patients lost to follow-up in each group, respectively. Intention-to-treat was used for data analysis, with n=21 in 750 mg Curcuma longa, n=22 in 1500 mg Curcuma longa, and n=23 in simethicone (Figure 1). The characteristics of the patients at baseline were similar across study groups (Table 1). However, in both Curcuma longa groups, patients were slightly overweight (BMI, 23.0-24.9 kg/m2), while in the simethicone group, patients were normal weight (BMI, 18.5-22.9 kg/m2). Participant characteristics, alongside all variables assessed, are available as Underlying data27,28.
| Characteristic | 750 mg Curcuma longa N = 27 | 1500 mg Curcuma longa N = 26 | Simethicone N = 25 | P-value |
|---|---|---|---|---|
| Female sex, n (%) | 19 (73.1) | 20 (76.9) | 18 (69.2) | 0.856 |
| Age, years* | 38.0±13.8 | 41.4±12.9 | 36.2±12.0 | 0.348 |
| Weight, kg* | 60.4±11.7 | 63.3±10.3 | 57.5±10.4 | 0.166 |
| Height, cm* | 158.0±8.2 | 159.3±7.1 | 160.3±7.6 | 0.551 |
| BMI, kg/m2* | 24.2±4.4 | 24.9±3.8 | 22.4±3.8 | 0.064 |
| Smoking, no (%) | 0.514 | |||
| Never | 25 (92.6) | 22 (84.6) | 20 (80.0) | |
| Former | 2 (7.4) | 2 (7.7) | 3 (8.0) | |
| Current | 0 | 2 (7.7) | 4 (16.0) | |
| Alcohol, n (%) | 0.169 | |||
| Never | 21 (77.8) | 24 (92.3) | 18 (72.0) | |
| Former | 1 (3.7) | 2 (7.7) | 2 (12.0) | |
| Current | 5 (18.5) | 0 | 4 (16.0) | |
| EGD approved FD, n (%) | 2 (7.4) | 2 (7.7) | 4 (16.0) | 0.517 |
| Duration of symptoms, years** | 3.0 (5.5) | 2 (4.0) | 2 (4.0) | 0.522 |
| Previous treatment, n (%) | 23 (85.2) | 24 (92.3) | 23 (92.0) | 0.627 |
| Global overall symptom scale* | ||||
| Epigastric pain | 4.2±1.5 | 3.5±1.6 | 4.0±1.9 | 0.283 |
| Heartburn | 2.4±1.5 | 2.3±1.5 | 2.8±1.9 | 0.492 |
| Upper abdominal bloating | 3.8±1.7 | 3.8±7.8 | 3.7±2.0 | 0.967 |
| Excessive belching | 3.2±1.8 | 2.5±1.7 | 2.8±1.7 | 0.369 |
| Nausea | 3.0±1.7 | 2.0±1.4 | 2.3±1.6 | 0.777 |
| Early satiety | 4.4±1.7 | 4.4±1.7 | 4.4±1.7 | 0.988 |
| Posprandial fullness | 5.6±1.0 | 5.5±1.3 | 5.5±1.2 | 0.906 |
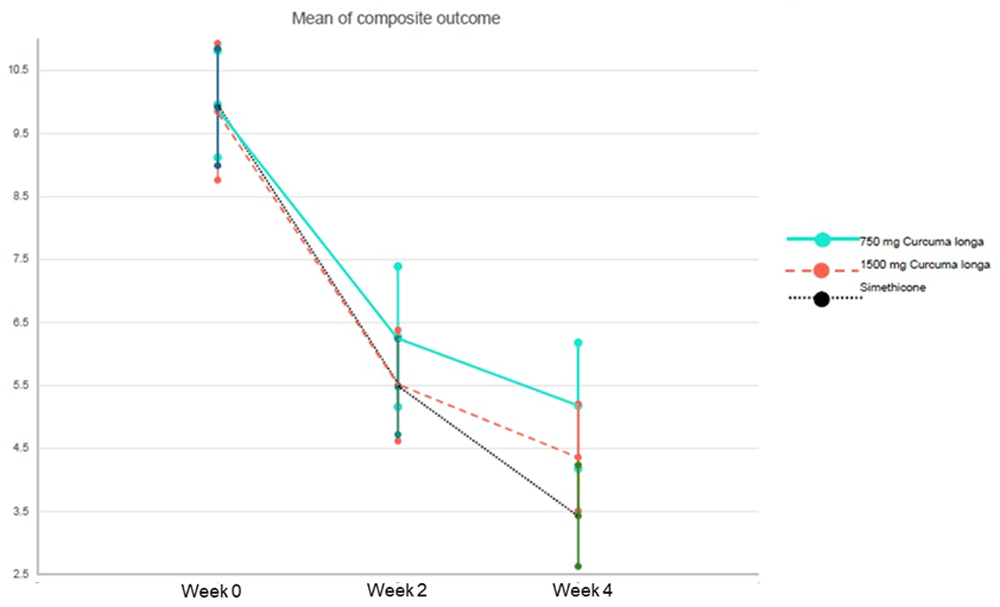
After 2 weeks, there was no significant difference in mean change of PDS symptoms among three groups [-4.1 (-4.5, -2.6) vs -4.3 (-5.2, -3.3) vs -4.2 (-4.8, -3.5), P=0.954]. Over a period of 4 weeks, patients who received simethicone, as compared with those who received Curcuma longa, had a greater reduction (improvement) in the composite outcomes of PDS symptoms, but there was no statistically significant difference [-4.6 (-5.7, -3.6) vs -5.4 (-6.6, -4.1) vs -6.2 (-7.2, -5.2), P=0.122] (Table 2).
Figure 2 shows the mean differences in GOS between three groups at the end of 2 and 4 weeks. When calculating mean differences of treatment effect between Curcuma longa groups and simethicone, there was no significant difference of treatment effect among two pair-wise comparisons (group 3 vs group 1 and group 3 vs group 2) at weeks 2 and 4 (Table 3).
| Comparison of treatment effect between groups at week 2 | P-value | |
|---|---|---|
| Treatment effect: mean differences in change (95% CI) | 0.954a | |
| Simethicone vs 750 mg Curcuma longa | -0.09 (-1.54, 1.36) | > 0.999b |
| Simethicone vs 1500 mg Curcuma longa | 0.09 (-1.40, 1.58) | > 0.999b |
| Comparison of treatment effect between groups at week 4 | P-value | |
| Treatment effect: mean differences in change (95% CI) | 0.122a | |
| Simethicone vs 750 mg Curcuma longa | -1.54 (-3.35, 0.28) | 0.123b |
| Simethicone vs 1500 mg Curcuma longa | -0.81 (-2.62, 1.00) | 0.828b |
Comparison of before and after treatment using the GOS scale at the end of 2 weeks. In Table 2, the 750 mg Curcuma longa group showed a significant reduction in all items of GOS scale except nausea which similar in simethicone group. However, in the 1500 mg Curcuma longa group indicated a significant reduction in almost all items of GOS scale except excessive belching and nausea.
Comparison of before and after treatment using the GOS scale at the end of 4 weeks. Over a period of 4 weeks, the participants among three groups showed significant improvement of their symptoms unless heartburn in 750 mg Curcuma longa, excessive belching and nausea in 1500 mg Curcuma longa, and nausea in simethicone (Table 2).
Rate of recurrence. After discontinuing treatment for 2 weeks (washout period), The patients with 1500 mg Curcuma longa reported the highest rate of recurrence, 45.5%, followed by the patients with 750 mg Curcuma longa, 42.9% and the lowest rate was in simethicone group, 13.6% (Table 4). In addition, the rate of symptom recurrence was found statistically significant among three groups (P=0.047).
| Rate or duration | 750 mg Curcuma longa (N=21) | 1500 mg Curcuma longa (N=22) | 240 mg Simethicone (N=22) | P-value |
|---|---|---|---|---|
| Recurrence patient -no. (%, 95%CI) | 9 (29.3%, 95%CI 3.7-54.8%)* | 10 (31.9%, 95%CI 6.5-7.1%)* | 3 | 0.047a 0.032b 0.020b |
| Mean duration of recurrence - day, mean (95%CI) | 4.1 (1.0, 7.2)** | 4.5 (1.0, 8.0)** | 4.2 (-6.1, 14.6)** | 0.984a |
Duration of recurrence. There was no significant difference in the duration of recurrence between groups (Table 4).
Adverse events. There was no any patient who needed to discontinue treatment due to the serious adverse events. Non-serious adverse events were reported in 8 cases (11.9%) from the patients who receive Curcuma longa, including nausea, diarrhea, fever, dizziness and headache (Table 5).
The main limitation of this study is that it is an open-label trial, in which blinding was not performed. There was no co-intervention, but few attrition biases. Although this is an open-label trial, we performed the allocation concealment and a good randomization that the results of similar characteristics among three treatment groups. Due to validity of the outcomes measured with precise 95% CIs in Table 3, our findings are summarizable and generalizable to all similar settings and populations.
The efficacy of Curcuma longa showed non-inferiority to simethicone according to the composite outcome of PDS symptoms among three treatment groups had no significant difference at week 2 and week 4. Our findings were similar to the findings of Sirijarugul and Pongchaidecha23 and Khonche et al24.
The study data also provide evidence of four important aspects of dyspepsia treatment. First, from baseline characteristics, there were more female participants. This is similar to the most recent meta-analysis in 20141. Data from this prior study indicated a greater prevalence of dyspepsia in the women from 312,415 samples (OR 1.24; 95% CI 1.13 to 1.36)1. The other characteristics were also accordant with previous studies23,24.
Second, our findings showed that Curcuma longa groups were also effective in different doses. Suprisingly, the 1500 mg group developed a higher symptom recurrence rate. Therefore, 750 mg Curcuma longa per day should be the recommended dose for FD.
Third, the simethicone group developed significant lower rate of symptom recurrence. To explain this phenomenon, these patients were normal weight from obesity Asian criteria (BMI, 18.5-22.9 kg/m2)29. On the other hand, in both Curcuma longa groups, patients were slightly overweight (BMI 23.0-24.9 kg/m2) that associated with the greater prevalence of GI symptoms30,31.
Finally, the three treatment groups were safe for all participants, similar to previous studies19,22–24, indicating that Curcuma longa can be used generally.
Strengths of this study were; this was a randomized controlled trial that had a high quality of evidence, we studied a washout period, and we are the first who compare the efficacy of Curcuma longa and simethicone. On the other hand, our limitations were; having lower sample size than calculated due to loss to follow up patients that might have less power of study and dyspepsia associated with the multifactorial factor such as environment, various types of food, and participant behaviors. Despite we randomly assigned the treatments, it could not eliminate all confounders. So the outcomes could be imprecise.
In conclusion, Curcuma longa had significant effects on reduction of FD, similar to simethicone after 2 and 4 weeks, but the recurrence rate (i.e. the proportion of reappearance) of dyspeptic symptoms was slightly significantly higher without serious adverse events.
Figshare: CurcumaUnderlyingData. https://doi.org/10.6084/m9.figshare.9962723.v127.
This project contains all de-identified variables assessed in this study.
Figshare: CurcumaDataDictionary. https://doi.org/10.6084/m9.figshare.1000062528.
This project contains the data dictionary for the underlying data, described above.
Figshare: CONSORT checklist for ‘Efficacy of Curcuma longa in treatment of postprandial distress syndrome: An open-label randomized-controlled trial’. https://doi.org/10.6084/m9.figshare.9962723.v127.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Authors appreciate all supports from Khon Kaen Hospital, patients, volunteers, first research assistant (Chutarat Tanchonnang) second research assistant (Manipa Jamsuwan) and all the staffs of all clinics involving in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiologist
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular epidemic, Immunogenetics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Oct 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)