Keywords
renal transplant, uroflowmetry, international prostate symptom score, overactive bladder symptom score
renal transplant, uroflowmetry, international prostate symptom score, overactive bladder symptom score
Chronic kidney disease (CKD) is a health burden affecting the global population, which has a high economic cost to health care, increases the risk of cardiovascular morbidity and decreases overall quality of life (QoL)1. The global prevalence of the five stages of CKD is between 11 – 13% with the majority of patients being stage 3, while stages 3–5 (end stage renal disease) is 10.6% (9.2 – 12.2%)1. Diabetes mellitus (DM) and hypertension (HT) is known to be significantly associated with CKD, with global prevalence of 8.5% in 20142 and 31% in 20103, respectively. According to the Basic Health Research in Indonesia (Riskesdas) in 20184, prevalence of DM and HT was 10.9% and 34.1%, however both non-communicable disease contribute to around 52% and 24% of people with CKD, respectively5. Previously, dialysis was thought to be the best treatment for patients with CKD, nowadays transplantation is a more attractive treatment due to its higher survival rates, QoL, cost effectiveness, and better clinical results6,7. On the other hand, patients with CKD who experienced prolonged oliguria and or anuria may develop bladder function complications and high intravesical pressure, known as neurogenic bladder, which may lead to renal impairment8. QoL might also be impacted due to obstruction and an overactive bladder, thus evaluation of bladder function is paramount after RTX9.
The aim of this study is to evaluate voiding characteristics of patients that have undergone RTX surgery in Jakarta, Indonesia.
In this prospective observational study, 71 patients diagnosed with CKD and had undergone a kidney transplantation from a living donor between January and December 2018 in Cipto Mangunkusumo General Hospital, Jakarta were examined. Total sampling method were implemented to include all potential subjects. Inclusion criteria for participants were consenting adults (> 18 years old) with sterile urine. Patients with neurogenic bladder and history of urinary obstruction were excluded.
RTX procedures were performed according to standardized techniques including a Gibson incision, extraperitoneal dissection, and end-to-side or side-to-side anastomosis of the donor to the recipient iliac vessels. Extravesical ureteral implantation (ureteroneocystostomy) was performed in an antireflux manner according to the Lich-Gregoir technique10. In all patients, a double-J stent was placed for 4 weeks and a transurethral catheter for the duration of hospital stay after operation.
Patients were informed regarding data collection before undergoing transplantation surgery. Pre and post-operative parameters were collected from medical record data. Voiding data (uroflowmetry), IPSS, and OABSS questionnaire was collected directly from the patients during follow up 1 month after the surgery.
Before transplant, anthropometric parameters, physical examinations, cause of CKD, daily urine production, types and duration of dialysis, and basic laboratory examination were collected (Table 1). Anuria and oliguria were defined as urine production of <100 cc/day and between 100 and 400 cc/day, respectively11.
Post-transplant, laboratory examination, international prostate symptom score (IPSS; male patients only), overactive bladder symptom score (OABSS), uroflowmetry, and post void residue (PVR) was gathered (Table 2).
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|
| Creatinine, mg/dL (mean ± SD) | 4.8 ± 2.27⊗ ** | 2.1 (0.4 – 10.1) ** | 1.4 (0.2 – 12.2) ** | 1.2 (0.6 – 13.1) ** | 1.2 (0.6 – 7.9) ** |
| Daily urine production, cc (mean, range) | 6150 (1530 – 16150) ** | 6428.3 ± 3035.7⊗ | 5636.3 ± 2185.9⊗ * | 5345.3 ± 1911.2⊗ | 4800.2 ± 1705⊗ * |
| Uroflowmetry (1-month post operation) | |||||
| Qmax, cc/s (mean ± SD) | 22 ± 9.8⊗ | ||||
| < 15 cc/s (n, %) | 21 (29.6) | ||||
| > 15 cc/s (n, %) | 50 (70.4) | ||||
| Voided volume (cc) | 208 (25 – 800) | ||||
| < 150 cc | 19 (26.8 %) | ||||
| > 150 cc | 52 (73.2 %) | ||||
| PVR, cc (mean, range) | 33.5 (2.3 – 142) | ||||
| < 100 cc (n, %) | 65 (91.5) | ||||
| > 100 cc (n, %) | 6 (8.5) | ||||
| IPSS (men only; 1-month post operation; n = 52) | |||||
| Frequency | 2 (0 – 5) | ||||
| Urgency | 0 (0 – 5) | ||||
| Nocturia | 2 (0 – 5) | ||||
| Irritative score | 5 (0 – 14) | ||||
| Incomplete emptying | 0 (0 – 5) | ||||
| Intermittency | 0 (0 – 4) | ||||
| Weak stream | 0 (0 – 2) | ||||
| Straining | 0 (0 – 5) | ||||
| Obstructive score | 0 (0 – 11) | ||||
| IPSS total score | 6 (0 – 21) | ||||
| Mild | 35 (67.3 %) | ||||
| Moderate – severe | 17 (32.7 %) | ||||
| QOL score | 1 (0 – 3) | ||||
| OABSS (1-month post operation) | |||||
| OABSS 1 | 1 (0 – 2) | ||||
| OABSS 2 | 2 (0 – 3) | ||||
| OABSS 3 | 0 (0 – 5) | ||||
| OABSS 4 | 0 (0 – 5) | ||||
| Total OABSS | 4 (1 – 13) | ||||
PVR: post-void residual; IPSS: international prostate symptom score; QOL: quality of life; OABSS: overactive bladder symptom score. Wilcoxon signed rank test was used for statistical analysis. ⊗ Normally distributed, paired T test was used for statistical analysis. * p <0.05, compare to previous value; ** p <0.001, compare to previous value.
Frequency, urgency, and nocturia were added and categorized as storage score, while the sum of incomplete emptying, intermittency, weak stream and straining were considered to be voiding score. Total IPSS score were classified into two groups: mild (score 0 – 7), and moderate-severe (score 8 – 35). A QoL question was also included as part of the IPSS questionnaire. The IPSS questionnaire was translated into Indonesian, reviewed and validated by the Indonesian Urological Association12. The sum score of daytime frequency, nocturia, urgency and urge incontinence made up the OABSS questionnaire with a maximum (worst) score of 2, 3, 5, and 5, respectively. A higher score indicated more severe symptoms with a maximum score of 15. A validated Indonesian version of this questionnaire was used13.
The maximum flow rate (Qmax) was measured with stationary uroflow recorder (Medtronic Urodyn® 1000 system), where <15 cc/s were associated with lower bladder outlet obstruction (BOO) (specificity of 38%, positive predictive value of 67%, and sensitivity of 82%)14. Consecutive urine voided >150 cc was accepted as valid14. PVR was measured after uroflowmetry examination with ultrasonography in supine position using an elliptical formula15. This examination was taken 4 weeks after RTX.
Spearman’s correlation was used to analyze the association between all continuous variables. Wilcoxon signed rank test was used for comparing medians in abnormally distributed and paired T test was used for comparing means in normally distributed continuous values. A P value of less than 0.05 and 0.001 were considered statistically significant and very statistically significant, respectively. All analyses were performed with commercial statistical software (SPSS version 23 for Macintosh; SPSS, Chicago, IL).
This research was conducted under ethical review and approval from The Ethics Committee of Faculty of Medicine, University of Indonesia corresponding to protocol number 17-07-0631 and ethical approval number 602/UN2.F1/ETIK/2017.
All participants included in this research provided written informed consent according to ethical guideline from The Ethics Committee of Faculty of Medicine, University of Indonesia.
Between January and December 2018, there were 107 RTX surgeries performed in Cipto Mangunkusumo Hospital, Jakarta, Indonesia. A total of 36 patients were excluded from this study due to various reasons (15 had previous surgeries for urinary tract obstruction, 2 had previous RTX surgeries, 4 had acute rejection, 2 had hyper-acute rejection and immediate allograft nephrectomies, and 13 patients did not give consent). Therefore, a total of 71 patients were included in this study (Table 1). The mean age of patients were 46 ± 17.9 years old with male and female ratio of 52:19, respectively.
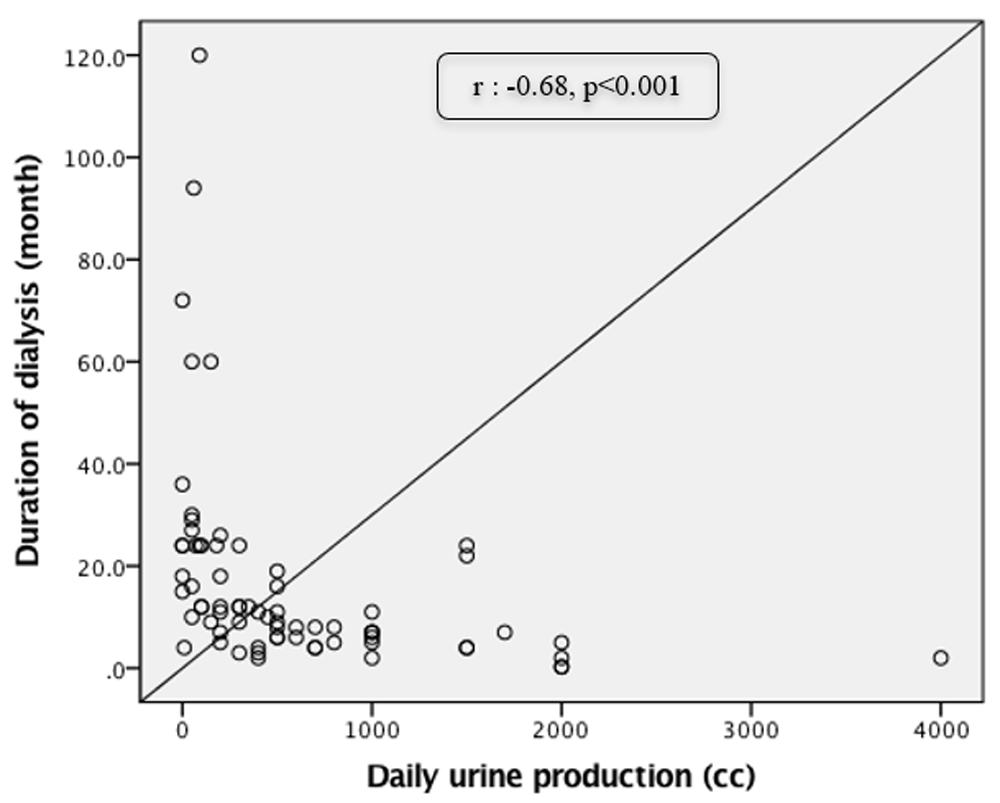
The major cause of CKD was HT (59.2%), followed by a combination of HT and DM (26.8%), other cause such as glomerulonephritis, nephrotic syndrome, polycystic kidney disease, and autoimmune disease (12.7%), and DM alone (1.4%). The mean preoperative creatinine was 5.86 ± 1.3 mg/dL. The majority of diabetic patients had controlled glucose level with median fasting blood glucose of 87 (72 – 242) mg/dL and HbA1c of 5.1% (4 – 8.2), according to the American Diabetes Association guidelines16. A total of 28% and 26.8% of patients had anuria (urine production <100 cc per day) and oliguria (urine production <400 cc per day), respectively. The main renal replacement therapy (RRT) used by patients was hemodialysis (98.6%) with median duration of 10 months [range, 0.3 (preemptive dialysis) – 120 months]. Significant negative correlation was seen between duration of dialysis and daily urine production (r: -0.68, p<0.01), as shown in Figure 1.
All postoperative variables are shown in Table 2. There are trends of decreased creatinine from the first day of 4.8 ± 2.27 mg/dL to 1.2 mg/dL (0.6 – 7.9) at day 5-post operation. The increase in urine production was also seen before and after the operation. During the first day, urine production varied between patients with a median of 6150cc (1530 – 16150) per day. However, from the second day onwards, urine production started to decrease from 6428.3 ± 3035.7 cc per day to 4800.2 ± 1705 cc per day at day 5. On uroflowmetry examination, the majority of patients had Qmax >15 cc/s (70.4%) with average flow of 22 ± 9.8 cc/s. Median voided volume was 208cc (25 – 800); 19 patients (26.8%) had insignificant voided volume (<150cc), which made the examination invalid. However, only 2 out of 19 patients had urinary retention (PVR >100 cc). The majority of patients had PVR <100 cc (91.5%) with median PVR of 33.5 cc (2.3 – 142). IPSS result shows that frequency [2 (0 – 5)] and nocturia [2 (0 – 5)], components of irritative symptoms, were the main problem in these patients with a total number of 20/52 (38.5%) and 40/52 (76.9%) patients, respectively. In total, 35 patients (67.3%) had only mild symptoms, with a median total IPSS score of 6 (0 – 21). Nevertheless, the majority of patients were “pleased” [1 (0 – 3)] on their QoL assessment. Similar to IPSS, the OABSS questionnaire showed that frequency (OABSS 1) and nocturia (OABSS 2) was the main symptoms reported by patient, with scores of 1 (0 – 2) and 2 (0 – 3), respectively.
RTX is thought to be a better alternative RRT compared to dialysis in terms of QoL, long-term survival, and financial burden6,7. However, a sudden increase in urine volume inside the bladder, a smaller bladder capacity, and bladder smooth muscle atrophy might cause irritative and/or obstructive symptoms, which in the long term might lead to decrease of graft function17–21. Low-pressure urine storage, effective bladder emptying, and minimal residual volume is necessary for maintaining sufficient and prolonged renal function.
Median daily urine production in our study was 400cc (0 – 4000) with 28.8% and 26.8% of patients having anuria and oliguria, respectively. Median duration of dialysis, 98.6% used hemodialysis, was 10 months (preemptive – 120). There was significant negative correlation between daily urine production and duration of dialysis (r: -0.68, p<0.01). This shows that longer duration of dialysis may decrease urine production, resulting in smaller bladder capacity8,17,19–24. Mean Qmax was 22 ± 9.8 cc/s, with 70.4% of patients exceeding 15 cc/s and valid examination (voided volume > 150 cc) in only 73.2% of patients. This result was not associated with gender (p>0.05). There was no definitive normal value of Qmax in women to determine BOO. However, a study by Mahfouz et al. shows that normal range of Qmax in asymptomatic women was 13 to 25 cc/s25. Therefore, in the present study we set the same threshold for Qmax between men and women to define BOO. To the authors knowledge, there are not many studies evaluating Qmax in patients after RTX; however, a study by Gratzke et al., concluded that in male patients with a long history of dialysis, early operative intervention due to urinary retention must be anticipated after RTX26. In our study, 6 patients (3 male and 3 female) had urinary retention (PVR > 100 cc) after uroflowmetry. Two patients had HT alone and 4 patients had both HT and DM as the cause of CKD. In both animal models and human, HT is shown to cause bladder dysfunction by altering beta-adrenergic receptor-mediated detrusor relaxation and alpha(1)-adrenergic receptor-mediated contraction of the bladder neck27.
In the present study, the main symptoms patients experienced after RTX was frequency of urination and nocturia with a total of 20/52 (38.5%) and 40/52 (76.9%) patients, respectively. This result was similar to other studies by Zermann et al.21 (frequency 87% and nocturia 94%), Van der Weide et al.20 (frequency 54% and nocturia 60%), and Lebadi et al.28 (nocturia 71.1%). Frequency might be due to thick-walled uncompliant high-pressure bladders, which might be caused by increased daytime production of urine, urinary tract infection (UTI) (common condition in post-transplant patients), and small bladder capacity (due to bladder atrophy)20. On the other hand, nocturia is mainly caused by increase in nighttime urine production (due to dysfunctional diurnal rhythm in urine production), large bladder capacity (which might lead to increase PVR and UTI), and decreased bladder compliance due to aging28. Both symptoms might also be caused by increased oxidative stress, which damages nerves, epithelium and smooth muscle of the bladder; however more studies are warranted. Similar results were shown with the OABSS questionnaire in the current study, which suggests patients after RTX have symptoms of mild-moderate overactive bladders. Regardless of this fact, the majority of patients’ QoL were not affected; 94.2% of all patients were pleased after RTX due to the fact that they do not need RRT, and a similar result was found by another study21.
In this study, one male older patient had decreased urine production (910 cc/day at day 3 to 375 cc/day at day 5) and increased creatinine (12.2 mg/dL at day 3 to 13.1 mg/dL at day 4, decreased to 7.9 mg/dL at day 5) starting from the third day after RTX, which suggested delayed graft function. This patient had left ventricular ejection fraction (LVEF) of 56%, chronic heart failure, and long term controlled HT. According to Rusinaru et al., renal dysfunction was found in around 14% of cases with heart failure (LVEF ± 50%), which is caused by intrinsic nephropathy, poor renal perfusion, vasoconstriction, and renal venous congestion29. Renal dysfunction is increased considerably with the degree of heart failure30. One month after surgery the patient had creatinine level of 1.9 mg/dL with normal Qmax and PVR of 33 cc/s and 35.4 cc, respectively. This proves that patients with decreased LVEF had increased risk for delayed graft and worse long-term graft function31.
Several limitations of this study were: 1) urodynamic evaluation was not used due to the limited financial and technical resource, 2) short duration of follow-up (1 month) was due to the variety of origin of transplant patients across Indonesia’s archipelago (difficult to maintain long-term follow up). In the future, prospective long-term follow up studies may be needed to precisely confirm our findings.
Daily urine production significantly correlates to duration of dialysis which, in the long-term, might alter lower urinary tract function. After RTX, the majority of patients experienced frequency and nocturia problems due to various causes, such as increased daytime and nighttime urine production, UTI, bladder capacity (both small and large), and decreased bladder compliance. Regardless of this fact, the majority of patients’ QoL were not affected; 94.2% of all patients were pleased after RTX due to the fact that they do not need RRT.
Harvard Dataverse: Voiding Profile in Recipients Post Renal Transplant, https://doi.org/10.7910/DVN/XGKFWV32
This project contains the following underlying data:
Harvard Dataverse: Voiding Profile in Recipients Post Renal Transplant, https://doi.org/10.7910/DVN/XGKFWV32
This project contains the following extended data:
Harvard Dataverse: STROBE checklist for ‘Voiding Profile in Recipients Post Renal Transplant’, https://doi.org/10.7910/DVN/XGKFWV32
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Nephrology and Transplantation
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Kidney Transplant.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 31 Oct 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)