Keywords
respiration, hypoxia, hypercapnia, ventilation
respiration, hypoxia, hypercapnia, ventilation
Postoperative respiratory complications commonly occur, with an incidence of up to approximately 10% in general surgery1–4 (even higher with thoracic surgery5). Complications include post-extubation hypoxemia, reintubation, acute respiratory failure, pulmonary edema, pneumonia, and atelectasis. These increase hospital length of stay, unplanned ICU admissions, hospital readmissions, mortality, and costs6–11. For example, respiratory failure after abdominal surgery can increase 30-day mortality 10-fold6.
Pathologically, we can characterize respiratory complications as being due to respiratory muscle dysfunction or as a primary airway disease. The latter can in turn be subdivided into upper airway-related complications, such as reintubation of an obstructive sleep apnea (OSA) patient, or pulmonary complications, such as pulmonary edema.
Both respiratory muscle dysfunction and airway disease can develop as a consequence of an imbalance in ventilatory drive. Both increases and decreases in ventilatory drive are potentially harmful and may, for example, increase the risk of aspiration by negatively affecting the interaction between breathing and swallowing (Figure 1). Sedation due to opioid and anxiolytic therapy commonly leads to upper airway dysfunction, resulting in insufficient respiration (hypopnea/apnea), but also affects the breathing–swallowing coordination and pharyngeal muscle strength, both of which contribute to pharyngeal dysfunction and increased risk of aspiration12. In turn, an increase in respiratory drive (e.g. during hypercapnic respiratory failure) can lead to high transpulmonary pressure during inspiration, which increases lung stress. Supplementation of inhaled carbon dioxide was shown to reverse upper airway collapsibility induced by propofol13, but excessive hypercapnia increases the likelihood of pathological swallowing14. Thus, perioperative physicians need to balance their interventions to keep ventilatory drive within normal limits. Upper airway collapse can lead to desaturation, atelectasis, and respiratory failure. Patency of the upper airway depends on competing dilating versus collapsing forces15,16. The former includes the pharyngeal dilator muscles (genioglossus and tensor palatini) and caudal traction on the airway from lung expansion (which can be improved by positive end-expiratory pressure [PEEP]). Sedatives, opioids, or even delirium can decrease airway dilator muscle tone. Dilating forces are influenced by atelectasis or the inevitable supine position of surgery. In contrast, collapsing forces include external pressure from surrounding soft tissue, which is increased in the presence of edema, obesity, blood clots, and tumors or in the supine position.

Changes in respiratory drive play a key role in the development of postoperative respiratory complications. Both increases and decreases in respiratory drive are potentially harmful and can affect the risk of aspiration. In addition, an increase in respiratory drive, for example during hypercapnic respiratory failure, can lead to high transpulmonary pressure during inspiration, which increases lung stress. Sedation commonly leads to upper airway dysfunction, resulting in insufficient respiration (hypopnea/apnea) but also affects the breathing–swallowing coordination and pharyngeal muscle strength, both of which contribute to pharyngeal dysfunction and increased risk of aspiration12. Supplementation of inhaled carbon dioxide was shown to reverse upper airway collapsibility induced by propofol13, but excessive hypercapnia increases the likelihood of pathological swallowing14. Thus, perioperative physicians need to balance their interventions to keep ventilator drive within normal limits. ARDS, acute respiratory distress syndrome.
Remarkably, perhaps, significant postoperative pulmonary edema is reported in up to 1–2% of patients9, and causes include negative pressure pulmonary edema, fluid shifts, and, rarely, neurogenic edema in acute hypertension or after cerebral injury17.
More common than edema is atelectasis, and its pathophysiology starts minutes after induction18. A reduced regional transpulmonary pressure in dependent lung areas is accentuated by inflammation induced by surgery, bacterial translocation, chest wall restriction, and cephalad diaphragm displacement by surgical retraction. This extends postoperatively, such that a restrictive pattern worsens respiratory mechanics and gas exchange. Pain, high inflation driving pressures, and inflammation all contribute.
Ventilator-induced lung injury has multiple causes. In addition to barotrauma, reduced lung compliance in unrecruited areas causes overinflation of aerated lung tissue in nondependent areas with subsequent “volutrauma”. Cyclical effects lead to “atelectotrauma”. As mentioned above, the release of local proinflammatory mediators also contributes to lung injury “biotrauma”19,20.
Modifiable perioperative factors in patient management are shown in Table 1. All the aforementioned pathophysiological processes make the optimization of ventilation as a protective strategy logical. What is really important, though, is preoperative screening and patient selection. The Score for Prediction of Postoperative Respiratory Complications (SPORC) is useful in this regard, as it relates the probability of re-intubation to ASA score, emergency surgery, heart failure, and pulmonary disease21. However, SPORC does not include factors such as smoking. Smoking is associated with increased risk of postoperative respiratory complications, and smoking cessation before surgery has been shown to decrease adverse respiratory events22,23.
| Factor | Main findings | Definition of PRC | Cohort | Reference |
|---|---|---|---|---|
| Case management | ||||
| Open vs. laparoscopic surgery | Laparoscopy reduced PRCs | Pulmonary infection, ARDS, symptomatic pleural effusion, respiratory insufficiency, pulmonary embolism | 1,214 patients undergoing major hepatectomy | Fuks et al.30 |
| General vs. regional anesthesia | Neuraxial anesthesia reduced mortality and PRCs | Pulmonary embolism, pneumonia, respiratory depression | 9,559 patients undergoing surgery with or without epidural or spinal anesthesia (systematic review) | Rodgers et al.31 |
| Ventilation | ||||
| Protective ventilation | Intraoperative protective ventilation was associated with lower risk of PRCs | Respiratory failure, reintubation, pulmonary edema, pneumonia | 69,265 non-cardiac surgical patients undergoing general anesthesia with endotracheal intubation | Ladha et al.28 |
| Case- specific PEEP | Reduced risk of PRCs and hospital length of stay with PEEP ≥5 cm H2O in abdominal surgical, but not craniotomy, patients | Respiratory failure, reintubation, pulmonary edema, pneumonia | 5,915 major abdominal surgical patients and 5,063 craniotomy patients | de Jong et al.29 |
| FiO2 | High intraoperative FiO2 was dose-dependently associated with PRCs and mortality | Respiratory failure, reintubation, pulmonary edema, pneumonia | 73,922 mechanically ventilated non-cardiac surgical patients | Staehr-Rye et al.32 |
| Pharmacological factors | ||||
| Volatile anesthetics | Higher doses of inhalational anesthetics were associated with lower risk of PRCs, reduced mortality, and reduced costs | Respiratory failure, reintubation, pulmonary edema, pneumonia | 124,497 non-cardiac surgical patients undergoing general anesthesia with endotracheal intubation | Grabitz et al.33 |
| NMBAs | Postoperative residual block (TOF ratio <0.7) after pancuronium administration was a risk factor for PRCs | Pneumonic infiltrations or atelectasis on chest X-ray | 691 patients undergoing abdominal, orthopedic, or gynecological surgery under general anesthesia | Berg et al.34 |
| Intermediate-acting NMBA use was associated with increased risk of PRCs | SpO2 <90% with a decrease after extubation of >3%, reintubation | 18,579 patients undergoing surgical anesthesia with NMBA use and 18,579 matched reference patients | Grosse-Sundrup et al.35 | |
| NMBA use (and neostigmine reversal) was dose-dependently associated with PRCs | Respiratory failure, reintubation, pulmonary edema, pneumonia | 48,499 non-cardiac surgical cases with NMBA use | McLean et al.36 | |
| NMBA use was associated with increased risk of PRCs | Respiratory failure, pulmonary infection, pulmonary infiltrates, atelectasis, aspiration pneumonitis, bronchospasm, pulmonary edema | 22,803 non-cardiac surgical patients undergoing general anesthesia | Kirmeier et al.37 | |
| Fluid management | Liberal fluid administration was associated with PRCs | Respiratory failure, reintubation, pulmonary edema, pneumonia (secondary outcome) | 92,094 non-cardiac surgical patients undergoing general anesthesia with endotracheal intubation | Shin et al.38 |
| Liberal fluid administration had a higher risk of pneumonia and pulmonary edema; goal-directed therapy had a lower risk of pneumonia | Respiratory failure, pulmonary edema, pneumonia, and pleural effusion (secondary outcome) | 5,021 surgical patients enrolled in 35 RCTs (meta- analysis) | Corcoran et al.39 | |
| Opioids | High intraoperative opioid dose was associated with increased readmission rate but not PRCs | Respiratory failure, reintubation, pulmonary edema, pneumonia (secondary outcome) | 74,748 surgical patients undergoing general anesthesia | Grabitz et al.40 |
| Most events occurred within 24 hours after surgery and were preventable in most cases | Respiratory depression | 357 acute pain claims | Lee et al.41 | |
| Opioids and sedatives are independent and additive predictors of the outcome | Cardiopulmonary and respiratory arrest | 6,771,882 surgical inpatient discharges | Izrailtyan et al.42 | |
The method of anesthesia induction can be preventative for postoperative complications. Keeping a patient as upright as possible during induction may help optimize mask ventilation and also help during extubation. This approach may prevent atelectasis, which may be especially important in patients with OSA24,25.
After intubation, lung-protective mechanical ventilation aims to maintain lung recruitment by keeping transpulmonary pressures within the optimal (linear) part of the local pressure–volume curve. Results from ICU patients suggest reduced morbidity and mortality in the setting of acute lung injury26,27. Typically, a PEEP of at least 5 cm H2O and a median plateau pressure of 16 cm H2O appear to be the most beneficial28. However, protective effects of PEEP may be very procedure specific, as a PEEP of approximately 5 cm H2O in major abdominal surgery is beneficial, whereas this is not matched by effects of the same level of PEEP in neurosurgery29. Also, PEEP must be patient specific: those with poor chest wall compliance need higher levels of PEEP43. Although high FiO2 is used to maintain oxygenation, it may also worsen pulmonary function, probably by promoting atelectasis32.
Interestingly, it has been found that an increased average minimum alveolar concentration of volatile anesthetics, including nitrous oxide, improves 30-day mortality and the risk of pulmonary complications33. The adverse influence of neuromuscular blocking agents (NMBAs) is now well established, especially when associated with inadequate reversal34–37,44,45. Monitoring of NMBAs along with reversal guided by neuromuscular transmission is now mandatory according to minimum monitoring guidelines in the UK46. The choice of reversal agent remains controversial; while sugammadex was shown to reduce the incidence of postoperative residual paralysis compared with neostigmine in one randomized controlled trial47, a recent multicenter observational study (POPULAR trial) found no association between the reversal agent used and postoperative respiratory complications37.
With regard to fluid administration, it is the most-restrictive and the most-liberal strategies that have been associated with respiratory complications, whereas moderate regimens appear to be optimal38,39,48. Pain is an adverse factor for respiratory complications, but very high doses of opioids are also potentially harmful40. Neuraxial blockade may reduce postoperative morbidity and mortality in subpopulations31,49, and laparoscopic surgery, which may contribute to better analgesia, further appears beneficial30. Good pain relief also promotes early mobilization, which shortens patients’ length of stay50. Monitoring is important in the detection of early signs of respiratory complications and the decision to admit and observe a patient in the ICU as opposed to the PACU51.
There is a considerable literature base supporting the individual results highlighted above. What is emerging is the need for the development and implementation of center-specific guidelines, based on algorithms, coupled with key performance indicators developed by multidisciplinary teams (Figure 2). This can form the basis of a continuous quality improvement program. An important driver in achieving this goal is a local champion or “facilitator”, who can lead the integration of the needed processes.
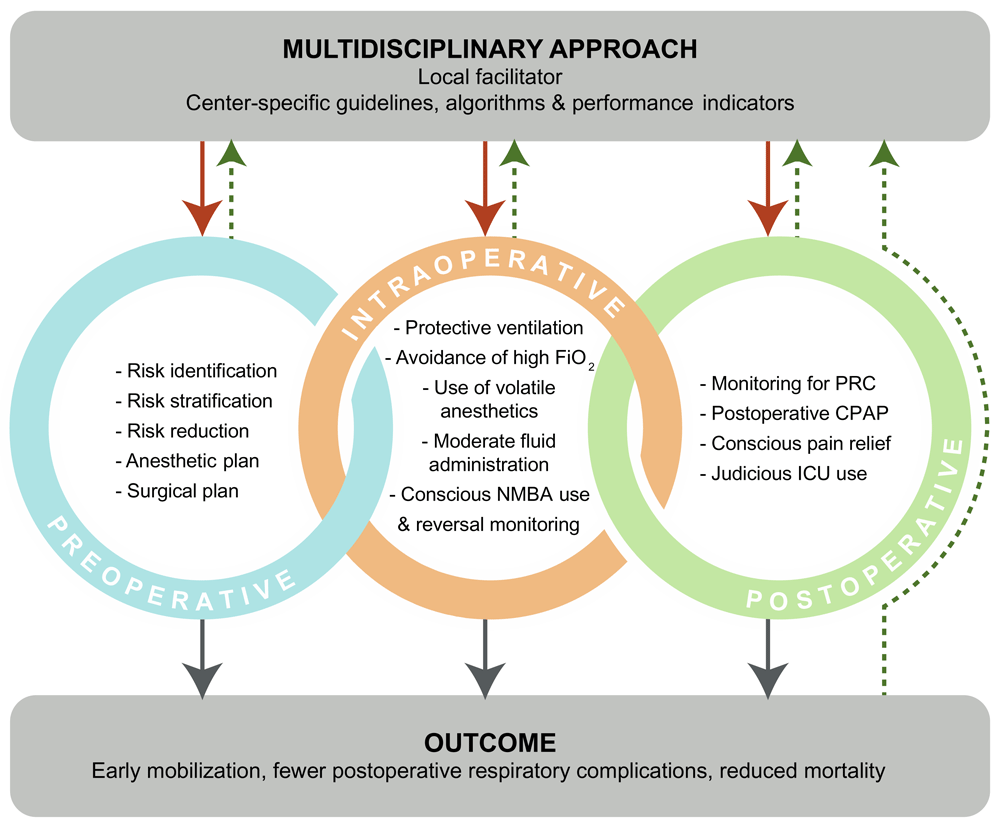
In a multidisciplinary approach, center-specific guidelines, algorithms, and performance indicators should be developed. Their implementation (red solid arrows) can be facilitated by a local “champion”. Factors concerning the preoperative, intraoperative, and postoperative period need to be addressed, as each can have an impact on outcomes. Periodic review and assessment of processes and outcomes (green dotted arrows) will ensure continuous improvement. CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; ICU, intensive care unit; NMBA, neuromuscular blocking agent.
This work was supported by an unrestricted grant from Jeff and Judy Buzen to develop personalized perioperative care to ME.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Feb 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)