Keywords
Furosemide, pancreatitis, Transurethral Resection of Prostate, Drug-Related Side Effects and Adverse Reactions
Furosemide, pancreatitis, Transurethral Resection of Prostate, Drug-Related Side Effects and Adverse Reactions
Acute pancreatitis caused after transurethral resection of the prostate (TURP) is an uncommon complication, with few reports found in the literature. It usually occurs in those who develop TURP syndrome1.
The prognosis depends on suspicion of this complication in a timely manner because it lead to multi-organ failure and even death1.
The case presented herein describes the case of a middle-aged patient who develops pancreatitis after TURP. The report describes how this was managed, the treatment used, and a review of the literature to identify similar cases.
A timeline of the patient’s case can be seen in Figure 1.
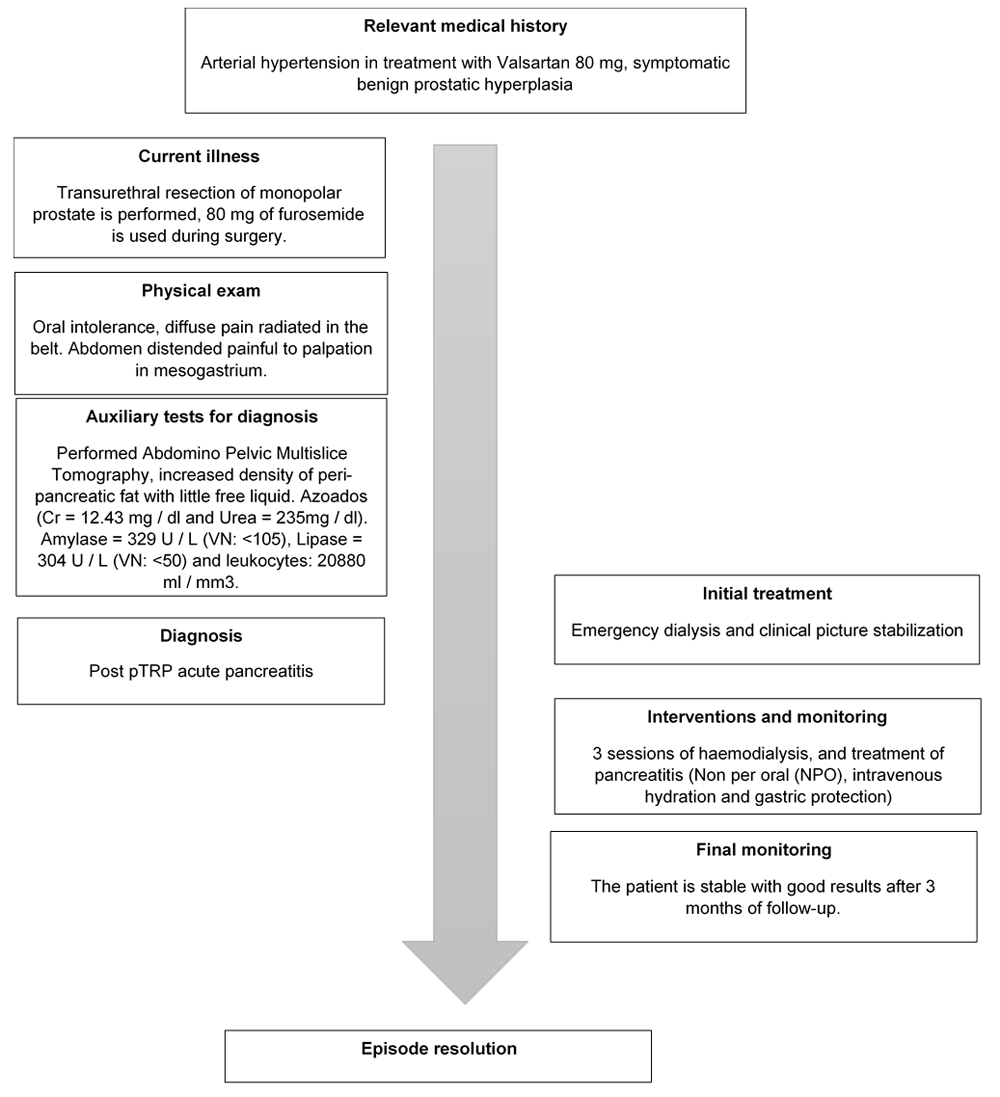
A 66-year-old male with a prior history of high blood pressure (treatment, Valsartan 80 mg bid), without any prior history of alcoholism, previous pancreatitis episodes, or gallbladder disease underwent TURP surgery after diagnosis with obstructive benign prostatic hyperplasia (BPH). This was detected with an approximate weight of 45 gr by ultrasound and a verum-neck distance of 3 cm in cystoscopy. The patient had no family history of hypertriglyceridemia or gallbladder stones.
Monopolar TURP surgery was performed using 13 liters of distilled water for continuous bladder irrigation. During surgery, 80mg of furosemide EV was used to reduce the risk of dilutional hyponatremia. The operative time was 55 minutes, removing 17gr of prostate tissue; with no intraoperative complications. No lesion of the capsule or any significant bleeding was reported.
After surgery, a physical abdominal and genitourinary exploration was carried out. There was evidence of oral intolerance, abdominal distention and increased abdominal resistance, with pain at the epigastrium that radiated to the lumbar region. Permeable Foley catheter with clear urine was reported.
At 24 hours post-surgery, the patient presented elevation of azoles (Cr = 3.7mg/dl; Urea = 92 mg/dl) and oliguria, therefore furosemide 20mg was administered twice a day. After 72 hours of follow-up, the condition persisted, and it was decided to increase treatment with furosemide to 20 mg EV three times a day; despite this, it evolves in an unfavourable manner. On the fifth day after surgery, elevation azoles continued (Cr = 12.43 mg/dl; Urea = 235mg/dl), and there was also an increase in pancreatic enzymes (Amylase = 329U/L(Normal value <105); Lipase = 304U/L(Normal value: <50) ; leukocytes = 20880ml/mm3 ( Normal value = 4500 ml/mm3 to 10500 ml/mm3). The aggregate infectious process was improved. An abdominal-pelvic CT scan was performed, finding a slight increase in the density of peripancreatic mesenteric fat with the presence of low laminar free fluid (Figure 2). The patient was transferred to the Intensive Care Unit (ICU) for emergency dialysis and stabilization.
Three sessions of haemodialysis and treatment of pancreatitis were required to stabilize the condition. On the fourth day of admission to the ICU, the patient progressed favourably from renal failure and pancreatitis by reduction in levels of pancreatic enzymes and creatinine, and on the 5th day the patient was discharged, tolerating oral feeding and stabilized renal failure.
Amoxicillin/clavulanic acid 500mg three times a day was prescribed for five days. The Foley catheter was removed on the fifth day after surgery as it was not necessary to monitor urine volume. Renal impairment was progressively restored, and the level of creatinine three months after surgery was 0.98mg/dl. No urethral stricture or any other urinary complications were reported.
Acute pancreatitis is an inflammatory disease of the pancreas that can also involve neighbouring tissues or organs, with a highly variable clinical presentation. In some cases, it has significant morbidity and mortality1,2; 20% of patients adopt a severe evolutionary course, with persistent organ failure leading to mortality in 25%. The reported age range is between 50 and 75 years, as the incidence of acute pancreatitis increases with age1,2.
Approximately 80–90% of cases are due to alcohol consumption and gallbladder lithiasis, while 10–20% have variable aetiology, including idiopathic, hyperlipaemias, viral infections, impaired pancreatic perfusion, ductal obstructions, drugs, and hypercalcemia3,4.
Drug-associated pancreatitis is an underreported entity with an incidence of 0.1 to 2%5. Drugs that have been associated with causing pancreatitis that have been reported in the literature include antihypertensive agents, proton pump inhibitors, anticonvulsants, and chemotherapeutic agents5.
Lesions in the pancreatic tissue is caused by aggressor factors (drugs, infection, or metabolic disorder) and by the secondary activation of digestive zymogens within acinar cells, which trigger a subsequent inflammatory response3. The mechanisms by which these complications develop are not fully understood, but intestinal endotoxins and inflammatory mediators play an important role1.
The diagnosis of drug-associated pancreatitis is by exclusion, so it is recommended to use the “Naranjo Scale of drug adverse reaction probability” in order to calculate the probability of adverse drug effect (Table 1). In 1981 Naranjo et al. validated this scale for cases of adverse drug reactions. In our case, the total score of our patient was 5 (answered affirmatively in questions 1, 2, 3 and 10), classified as probable in relation to furosemide6.
Drugs such as furosemide have been described that could damage pancreatic perfusion by decreasing intravascular volume, affecting blood flow, producing ischemia and the subsequent development of acute pancreatitis. It may also stimulate the exocrine pancreas producing a hypersensitivity reaction1,6,7.
In our patient, Valsartan was suspected of having contributed to the presentation. However, due to the prolonged exposure time, it was dismissed as a possible aetiology.
In the literature, risk factors associated with drug pancreatitis have been described, of which our patient presented advanced age and vascular risk factors. As a result of these factors an increase in pancreatic toxicity may have occurred, due to atherosclerosis of mesenteric vessels, resulting in furosemide making the perfusion worse by diuresis and intravascular volume depletion8. However, the exact mechanism is not yet known. However, some hypotheses include that furosemide could stimulate exocrine secretion of the pancreas, or mechanisms of hypersensitivity or immune response against a drug-protein. As evidenced in multiple reports, there are many confounding factors, but they only increase pancreatic susceptibility. In our case, the precipitating event is the administration of furosemide.
The present patient did not present capsule perforation or post-surgery bleeding. Berber-Deseusaa et al. present the case of a patient who presents capsule perforation during the intraoperative period1. In our case, pancreatitis was mild, presenting improvement on the eighth post-surgery day after dialysis. However, the case reported by Berber-Deseusaa et al. presented severe necrotizing pancreatitis, leading to the death of the patient1.
Acute pancreatitis is an infrequent complication reported after TURP1,9–11; it can delay diagnosis if it is not considered as a possibility1. In our patient, the surgery was without complication, unlike previous cases presented in the literature where capsule perforation was observed1. The present surgical time was 55 minutes.
On the other hand, the evolution of our patient was favourable in contrast to what was presented by Berber et al., where the two patients presented unfavourable evolution with acute renal failure, ventilatory-circulatory deterioration, and death1.
The clinical features of pancreatitis following TURP surgery has been reported previously in the literature but infrequently. This condition should be suspected when dealing with pain in the epigastrium post TURP. If this complication is suspected, imaging tests and pancreatic enzyme levels should be immediately requested, as it can have an unfavourable evolution until death.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Urology
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
No
References
1. PONKA JL, LANDRUM SE, CHAIKOF L: Acute pancreatitis in the postoperative patient.Arch Surg. 1961; 83: 475-90 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: endourological management of kidney stone and enlarged prostate
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 03 Dec 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)