Keywords
renegeration, pluripotent stem cells, kidney
renegeration, pluripotent stem cells, kidney
Establishment of human induced pluripotent stem (iPS) cells was a big step that brought us closer to a realization of organ regeneration and transplantation, which was a distant dream for many researchers1. Although the advancements in renal regeneration are falling behind in terms of technology compared with regeneration of other organs because of its complex structure, the strategy to induce renal three-dimensional (3D) structures in vitro has recently been advanced by the detailed analysis of developmental origins of the kidney. The National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD, USA) is now leading a consortium called “(Re)Building a Kidney” to optimize approaches for the isolation, expansion, and differentiation of appropriate kidney cell types and the integration of these cells into complex structures that replicate human kidney function2, which shows the extent of attention being given to this area of research. In this review, we summarize the recent advances and future perspectives of renal regeneration.
Accurate understanding of the organogenesis process is crucial for achieving renal regeneration. Three primordia—pronephros, mesonephros, and metanephros—are developed during embryogenesis3. Kidneys are derived from the embryonic metanephros, which develops at the most posterior part of the body trunk, whereas the pronephros and mesonephros develop at the more anterior part of the body trunk in earlier developmental stages, which eventually degenerate. Metanephros is formed by three progenitor cells: nephron progenitors, ureteric bud, and stromal progenitors. Nephron progenitors undergo mesenchymal-to-epithelial transition, forming glomeruli and renal tubules; ureteric bud undergoes branching morphogenesis, forming collecting ducts and ureters; and stromal progenitors differentiate into interstitial cells. Thus, the optimal induction of these three progenitor cells from pluripotent stem cells (PSCs) is a critical step in achieving renal regeneration4. Moreover, the interaction between these three progenitors is important (Figure 1). The tips of the ureteric bud (UB tips) send signals to maintain undifferentiated nephron progenitors and induce mesenchymal-to-epithelial transition of nephron progenitors by a transient Wnt signaling. In turn, the undifferentiated nephron progenitors produce glial cell–derived neurotrophic factor (Gdnf) to maintain UB tip proliferation, and the stromal progenitors support ureteric branching by retinoic acid signaling. These interactions attain nephron progenitors’ maintenance and differentiation at the same time, producing millions of nephrons with systemic connections. Therefore, the continuous supply of nephron progenitors5 and ureteric branching as a result of interactions between three progenitors4 are essential for organ-scale kidney morphogenesis.
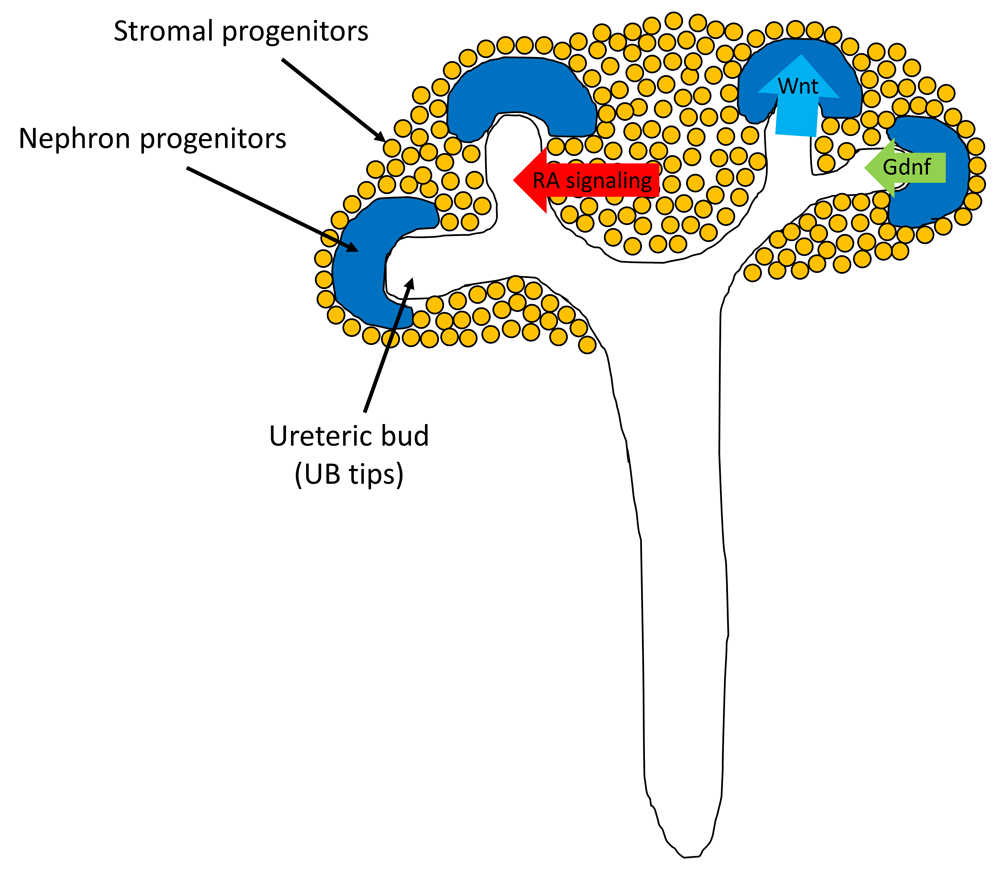
Nephron progenitors, ureteric bud (UB), and stromal progenitors interact with each other to undergo kidney organogenesis. Tips of the UB (UB tips) send signals to maintain undifferentiated nephron progenitors and induce mesenchymal-to-epithelial transition of nephron progenitors by transient Wnt signaling. In turn, the undifferentiated nephron progenitors produce glial cell–derived neurotrophic factor (Gdnf) to maintain UB tip proliferation, and the stromal progenitors support ureteric branching by retinoic acid (RA) signaling.
Both nephron progenitors and ureteric bud were believed to be derived from the same intermediate mesoderm, which appears on about embryonic day 8.5 (E8.5) and expresses paired box gene 2 (Pax2)6,7. Although the intermediate mesoderm-like cells have been successfully induced from PSCs8–10, the 3D structure of kidneys could not be regenerated from it. There is a possibility that the induced cells were not intermediate mesoderm but lateral plate mesoderm, for Mae et al. have not investigated the possibility of lateral plate mesoderm induction in their generating cells although they used odd skipped–related 1 (Osr1) as the selection marker8, which was thought to be specifically expressed in the intermediate mesoderm but, in fact, is also expressed in other areas such as lateral plate mesoderm. The cell-lineage tracing analysis11 recently revealed that precursor of nephron progenitors, which is T (Brachyury)-positive, is maintained and localized posteriorly in the undifferentiated state (posterior nascent mesoderm) until E8.5 and differentiates into posterior intermediate mesoderm at E9.5, which subsequently becomes nephron progenitors (Figure 2). This, however, is against the conventional concept which states that the entire kidney is derived from early-stage intermediate mesoderm which overlaps with recently recognized anterior intermediate mesoderm. Thus, nephron progenitors are different from ureteric bud in both their origin (nephron progenitors originate from posterior/late-stage intermediate mesoderm, whereas ureteric bud originates from anterior/early-stage intermediate mesoderm) and the timing of intermediate mesoderm differentiation (nephron progenitor lineage differentiates on E9.5, and ureteric bud lineage differentiates on E8.5). Owing to this detailed understanding of the kidney development process, Taguchi et al. succeeded in inducing nephron progenitor cells from mouse embryonic stem (ES) cells and human iPS cells via posterior nascent mesoderm and posterior intermediate mesoderm (Figure 3)12. They succeeded in maintaining the immature mesoderm state (T-positive) during the posteriorization phase by using an unusually high concentration of Wnt agonist. Subsequently, graded attenuation of the Wnt agonist, as well as stage-specific addition of growth factors, led to metanephric nephron progenitor formation. Moreover, they demonstrated that the induced nephron progenitors reconstituted the 3D structure of kidneys in vitro, including glomeruli with podocytes and renal tubules with proximal and distal regions12.
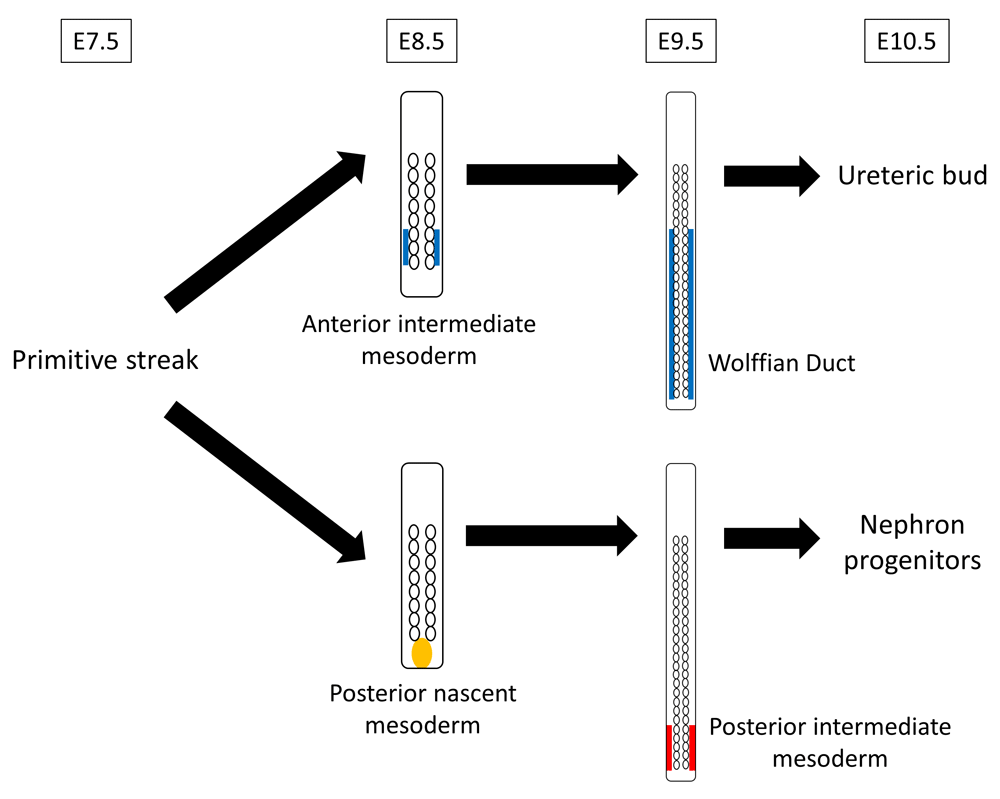
The precursor of ureteric bud is differentiated into anterior intermediate mesoderm on embryonic day 8.5 (E8.5) and forms the Wolffian duct on E9.5. The precursor of nephron progenitors is maintained and localized posteriorly in an undifferentiated state (posterior nascent mesoderm) until E8.5, differentiates into posterior intermediate mesoderm on E9.5, and subsequently becomes nephron progenitors.

Considering that the precursor of nephron progenitors is maintained and localized posteriorly in an undifferentiated state (posterior nascent mesoderm) until embryonic day 8.5 (E8.5), Taguchi et al.12 used an unusually high concentration of Wnt agonist to maintain the immature state during the posteriorization phase. Subsequently, graded attenuation of the Wnt agonist, as well as stage-specific addition of growth factors, led to metanephric nephron progenitor formation. The required signaling for the lineage specification at each embryonic stage (E) and the in vitro differentiation timing (Day) of human induced pluripotent stem (iPS) cells are shown4,12. Bmp4, bone morphogenetic protein 4; Fgf9, fibroblast growth factor 9; RA, retinoic acid.
Based on this concept, Morizane et al. successfully developed a more efficient method for inducing nephron progenitor cells13. Furthermore, some researchers have developed a method for selectively expanding nephron progenitors derived from mouse embryos, mouse ES cells, and human iPS cells5,14,15, which would help to reconstruct kidneys in vitro because the continuous supply of nephron progenitor cells is essential.
Proper organization of ureteric branching morphogenesis is essential for kidney function. Takasato et al. generated kidney organoids by induction of multiple kidney components, including nephron progenitors, ureteric bud, and stromal progenitors, using a single protocol16, but their organoids did not have a dichotomously branching ureteric tree. Taguchi and Nishinakamura induced ureteric bud from mouse ES cells and human iPS cells by using a protocol completely different from that used for the induction of nephron progenitors4. Since the duration of immature mesoderm state is different between nephron progenitors and ureteric bud, they regulated it by changing the exposure time to the Wnt signaling4,17. Furthermore, preparing a budding ureteric structure with an isolated E11.5 metanephric mesenchyme (including nephron progenitors and stromal progenitors) resulted in the dichotomous branching up to six or seven generations of ureteric bud with nephron progenitors on each ureteric bud tip (nephron progenitor niches) and formed differentiated nephrons containing distal and proximal tubules along with glomerular structures, suggesting how important it is to control the spatial arrangement of progenitor cells. This higher-order kidney organoid (branching ureter with nephron progenitor niches and differentiated nephron components) could also be reconstructed by adding separately induced nephron progenitors and ureteric bud from mouse ES cells to stromal progenitors sorted from E11.5 embryonic kidneys.
As mentioned above, rapid and remarkable advances have been made in the field of kidney regeneration, but reconstructing functional and sophisticated kidneys from PSCs is still a challenging task.
First, signals required for induction of the stromal progenitor lineage remain to be elucidated, even though the selective induction of the other two renal progenitors (nephron progenitors and ureteric bud) from PSCs has already been established. Kobayashi et al. revealed that Foxd1-expressing cortical stroma represents a distinct multipotent self-renewing stromal progenitor population that gives rise to stromal tissues using cell-fate mapping analysis18. The detailed differentiation process of stromal progenitors, however, has not yet been clearly understood. Thus, Taguchi and Nishinakamura still used stromal progenitors sorted from mouse embryonic kidneys when reconstructing higher-order kidney structures with separately induced nephron progenitors and ureteric bud from PSCs4. Since primary human embryonic stromal progenitors are not readily available, induction of stromal progenitors from PSCs is strongly required. Although Takasato et al. have reported that multiple kidney components, including stromal progenitors, can be induced from PSCs by a single protocol16, this kidney organoid has limited functionality and no ureteric bud cell types were detectable by single-cell RNA sequencing19. Given that nephron progenitors and ureteric bud have distinct origins, selective induction of the three renal progenitors from PSCs is one of the best strategies for reconstructing higher-order kidneys at this stage. Therefore, it is critical to understand the differentiation process of stromal progenitors and establish the optimal conditions for their induction from PSCs for the reconstruction of sophisticated and functional kidneys in vitro.
Second, at present, the reconstructed kidney organoid has not fully matured in scale, structure, and function. Although Taguchi and Nishinakamura were successful in reconstructing higher-order kidney organoid (branching ureter with nephron progenitor niches and differentiated nephron components), cDNA microarray analyses of their reconstructed kidney organoids revealed that their organoids cultured for 7 days most closely resembled the E15.5 kidney4, indicating that they were different from adult kidneys. It was speculated that blood supply might be required to make the organoids function as kidneys. Takebe et al. showed that liver buds generated from human iPS cells developed into vascularized and functional human livers by murine intracranial or mesenteric transplantation20, showing that blood perfusion is important for making reconstructed organs functional. Sharmin et al. transplanted human iPS cell–derived nephron progenitors beneath the kidney capsule of immunodeficient mice and demonstrated that the human glomeruli were vascularized by the host endothelial cells, resulting in further maturation of podocytes21. Van den Berg et al.22 have also shown that renal subcapsular transplantation in mice induces vascularization with blood perfusion of human iPS–derived kidney organoids reconstructed by Takasato’s protocol23, resulting in progressive maturation of nephron structures such as podocyte foot processes and polarization and segmental specialization of tubular epithelium. Although the transplantation approach by Sharmin et al. required the addition of vascular endothelial growth factor (VEGF) to the transplant21, Van den Berg et al. have shown that the kidney organoids themselves actively secrete VEGF and induce host-derived angiogenic vascularization after transplantation22. However, the vascular system formed in the transplanted kidney organoids is simple at this stage and there is still a very long way to go before the appropriate kidney vascular networks can be reproduced.
Furthermore, using kidney organoids as disease modeling might contribute to the medical research. Cruz et al. have generated a genetic model of polycystic kidney disease using human iPS cells and established a highly efficient model of cystogenesis24. Hale et al. have shown that podocytes in kidney organoids have improved podocyte-specific gene expression and polarized protein localization compared with podocyte cell lines cultured in 2D25. Tanigawa et al. established kidney organoids using human iPS cells from a patient with an NPHS1 missense mutation, identifying impaired nephrin localization and slit diaphragm formation in podocytes26. In this context, how to standardize the differentiation efficiency among the PSCs which have a different genetic background is a significant challenge. Czerniecki et al. recently presented a protocol for the miniaturization and automation of human organoid differentiation from iPS cells, showing that kidney organoids can be applied to high-throughput screening focusing on therapeutic discovery and toxicology27.
Application of recent technological advancements in human regenerative medicine can help in regenerating complex spatial arrangement of kidneys with vascular network and urinary excretion pathway.
One promising strategy might be a bioengineering technique such as decellularization and 3D bioprinting. Song et al. decellularized rat kidneys by detergent perfusion, which yielded acellular scaffolds, and then seeded them with epithelial and endothelial cells and perfused these cell-seeded constructs in a whole-organ bioreactor28. Although transplantation of this bioengineered kidney exhibited excretory function in vivo, optimization of cell-seeding protocols and upscaling of biomimetic organ culture are still required for their use in clinical settings. Application of 3D bioprinting methods has also been successful in reconstructing the complicated structures of proximal tubules29 and vasculatures30,31 in vitro, although the physiological functions reproduced by these technologies reflect only a small part of organs.
Another promising strategy might be regenerating human kidneys in other species. Kobayashi et al. first generated rat pancreas in mouse via interspecific blastocyst complementation32. Yamanaka et al. applied this concept and succeeded in regenerating rat-derived nephrons in mice by combining the transplantation of rat-derived nephron progenitors with the native nephron progenitors’ conditional elimination33, thereby demonstrating a technical platform for regenerating kidneys in other species. The same group was successful in demonstrating a stepwise peristaltic ureter system for constructing the urinary excretion pathway in stem cell–generated embryonic kidneys34. Concretely, rat metanephroi with bladders developed from cloacas were transplanted into host rats and then were connected to the host animal’s ureter (a stepwise peristaltic ureter system). Thus, functional kidneys can be theoretically reconstructed by the combination of three technologies—induction of nephron progenitors from human iPS cells, regeneration of human nephrons in other species, and construction of a urinary excretion pathway—which might be the most promising strategy for regenerative medicine at present. However, there is still a big ethical problem regarding the generation of chimeric animals.
Although regeneration of a functional kidney is difficult because of its complex structure, recent advancements in this field are remarkable. The cell-lineage tracing analysis has revealed details of the developmental process of renal progenitors, which allows the induction of two of the three renal progenitor cells from PSCs and the reconstruction of higher-order kidney organoids in vitro, even though the degree of maturation of these organoids is not satisfactory. Combining the induction of renal progenitors from PSCs with new bioengineering methods, including decellularization and 3D bioprinting, and the recent advancements in the regeneration of kidneys in other species would be a promising strategy for regenerating functional human kidneys.
This work was supported by Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI grant 18H02824 to MN), Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI grant 17K09688 to TT), and Grants-in-Aid for Scientific Research on Innovative Areas (KAKENHI grants 26111003 to MN).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Feb 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)