Keywords
Silver nanoparticles, Antibacterial, Self-etch adhesive, Streptococcus mutans, Minimum inhibitory concentration, Degree of conversion.
This article is included in the Nanoscience & Nanotechnology gateway.
Silver nanoparticles, Antibacterial, Self-etch adhesive, Streptococcus mutans, Minimum inhibitory concentration, Degree of conversion.
Dental resin composite is a widely used restorative material that has superior aesthetic properties and strong bonding ability to the tooth structure in comparison to other restorative materials like amalgam1. In spite of the recent advances in the dental adhesives, there is a high possibility of microbial biofilm development at the resin restoration surface, which may lead to marginal gap and recurrent caries2.
Silver is an antimicrobial agent that has broad spectrum activity against gram positive and gram negative bacteria2. Nanoparticles are insoluble particles smaller than 100 nm. Their unique size provides higher surface area, thus much more potent antimicrobial activity in comparison to the usual particles size3. Silver nanoparticles can cause cell membrane disruption and damage of the bacterial DNA1. Degree of conversion of the dental adhesive represents a relative assessment to its quality and directly correlates with its mechanical behavior. Proper polymerization of the dental adhesive can increase the longevity of the bonded restoration4. Therefore, it seems valuable to investigate the minimum inhibitory concentration of silver nanoparticles incorporated in two forms into the self-etch adhesive system and their effect on the degree of conversion.
The materials, preparations, manufacturers, composition and batch numbers are listed in Table 1.
1ml of ethanolic solution of silver nanoparticles was added to 5ml of self-etch adhesive system and serially diluted. To produce the adhesive system with the nano-silver powder incorporated, it was accurately weighed first using a scale (AE Adam, Bradford, UK). The previously measured powder (1000 µg) was added to 1 ml of adhesive solution, sonicated in an ultrasonic mixer (Eumax model: UD100SH-3LQ, China) and serially diluted using a micropipette (The final concentration of nanosilver in the adhesive resin in the two forms is 100µg/ml). The procedure was completely performed in a dark environment using X-ray processing box.
The minimum inhibitory concentration (MIC) defined as the lowest concentration of nanosilver in micrograms per milliliter (μg/ml) that inhibits the growth of an organism5. Tryptic soy agar medium; culture nutrient media for Streptococcus mutans (ATCC 25175) was poured in 10 petridish plates in a laminar flow (Telstar BIO- II-A, VWR Company, UK.). Streptococcus mutants strains; ATCC 25175 (Cairo MIRCEN, Faculty of agriculture, Ain Shams University, Egypt.) were cultured on Tryptic soy agar medium at 37°C for 24 hours6.
10 petridish plates were punched by a cork-borer with a 6 mm diameter to produce rounded holes in each plate and the bacterial strain was applied equally on the agar plates7,8. The specimens were grouped as N1: Self-etch adhesive system + nanosilver solution and N2: Self-etch adhesive system+ nanosilver powder. Each agar plate contained 5 holes representing the five different concentrations of the nanosilver. For N1 and N2 groups; the self-etch adhesive system was serially diluted to produce 5 subgroups with different concentrations, C1: 100 µg/ml, C2: 50 µg/ml, C3: 25 µg/ml, C4: 12.5 µg/ml and C5: 6.25 µg/ml. The adhesive system without nanosilver incorporation was used as a control group (N0).
200 µl of the adhesive agent was injected in each hole by micropipette and polymerized for 20 seconds using light emitting diode unit (Woodpecker Medical Instrument Company, Model LED. F; Model No. L14A0116F, China.) (Figure 1). The plates were incubated for 48 hours in the incubator at 37°C under completely anaerobic conditions (Shell lab Company, SMI6, Canada). The diameter of bacterial inhibition zone halo of each adhesive was measured in millimeters using a ruler7.
The specimens were prepared using a cylindrical Teflon mold surrounded by metallic ring (3mm diameter and 2mm height)9 (Figure 2). The mold was placed on a Mylar strip (Universal strips of acetate foil, Germany) that was placed on a clean flat glass slab. 200 µl of self-etch adhesive resin was injected in the hole of the mold using a micropipette then covered with the Mylar strip (to avoid the presence of oxygen inhibiting layer and pressed to obtain a uniform smooth specimen surface)4,9. The adhesive resin was cured in the presence of the top Mylar strip by LED device for 20 seconds according to manufacturer instructions. The light curing tip was applied perpendicular and with intimate contact with the top surface of the Mylar strip (Zero distance)4.
15 disc shaped specimens were prepared of self-etch adhesive system. The specimens were divided into 3 equal groups (n=5) according to the form of incorporated nanosilver (N0: Adhesive system without nanosilver, N1: Adhesive system+ nanosilver solution, N2: Adhesive system+ nanosilver powder). The concentration of the incorporated nanosilver was set according to the minimum inhibitory concentration (MIC) that was tested before.
Degree of conversion was measured using an Attenuated Total Reflectance/ Fourier Transform Infra-Red spectrometer (ATR/ FTIR) (Vertex 70, Bruker Company, Germany)4,10. All the data were recorded and plotted on a special computer software (OPUS Bruker Spectroscopy Software, version 7, Germany) to draw the linear graphs from which the degree of conversion of each specimen was calculated.
Data statistically was described in terms of mean values and standard deviation (SD) using ANOVA test (IBM® SPSS® (SPSS Inc., IBM Corporation, NY, USA, Statistics Version 22 for Windows).
Mean ± SD measures of the diameter of inhibition zone (DIZ) were summarized in Table 2 and Table 3 and graphically drawn in Figure 3 (underlying data available from OSF11). The largest zone of inhibition was at 100 µg/ml concentration of nanosilver powder while the smallest was at 6.25µg/ml concentration of nanosilver solution.
| Concentration of nanosilver in adhesive system | Nanosilver solution (N1) | Nanosilver Powder (N2) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| C1: 100 µgm/ml | 20.00 ± 1 | 22.00 ± 0.7 |
| C2: 50 µgm/ml | 15.20 ± 0.84 | 18.80 ±0.45 |
| C3: 25 µgm/ml | 14.80 ± 0.45 | 17.20 ±0.84 |
| C4: 12.5 µgm/ml | 13.80 ± 0.45 | 15.60 ±0.55 |
| C5: 6.25 µgm/ml | 10.40 ± 0.55 | 12.00 ± 0.55 |
| Control (N0): 0 µgm/ml | 11.60 ± 1.35 | 11.60 ± 1.35 |
| P value | ≤0.001* | ≤0.001* |
| Concentration of nanosilver in adhesive system | Nanosilver solution (N1) | Nanosilver Powder (N2) | P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | 0.006* | |
| C1: 100 µgm/ml | 20.00 ± 1 | 22.00 ± 0.7 | ≤0.001* |
| C2: 50 µgm/ml | 15.20 ± 0.84 | 18.80 ±0.45 | ≤0.001* |
| C3: 25 µgm/ml | 14.80 ± 0.45 | 17.20 ±0.84 | ≤0.001* |
| C4: 12.5 µgm/ml | 13.80 ± 0.45 | 15.60 ±0.55 | ≤0.001* |
| C5: 6.25 µgm/ml | 10.40 ± 0.55 | 12.00 ± 0.55 | 0.006* |
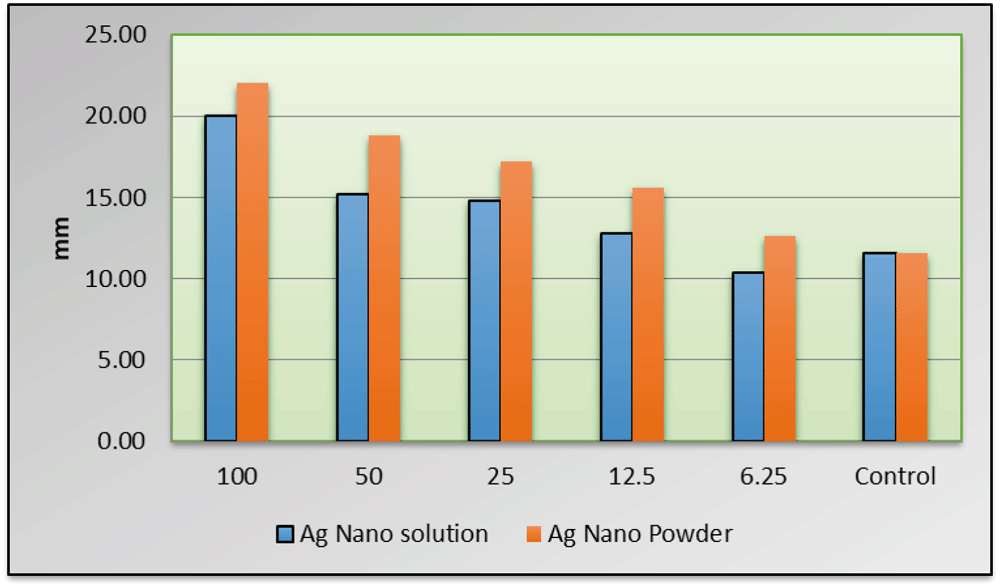
All the concentrations recorded significantly higher DIZ when compared to the control group (P≤0.05)except C5 ; 6.25µg/ml. The MIC of adhesive containing nanosilver (powder and solution form) was determined at 12.5 µgm/ml concentration. All the concentrations of nanosilver powder recorded significantly higher mean values of DIZ when compared to the different concentrations of nanosilver solution.
(Mean ± SD) measures of degree of conversion in % were summarized in Table 4 and graphically presented in Figure 4. Groups of adhesive system with incorporated nanosilver solution (26.14±4.47 %) recorded a statistically significant lower degree of conversion when compared to control (50.31±4.04 %) and nanosilver powder (47.72±4.47%) (P ≤ 0.001). There was no significant difference in the degree of conversion between the control group and the group of the adhesive system containing nanosilver powder (p≥0.05).
| Variables | Mean± SD | Rank | P value | |
|---|---|---|---|---|
| Nano-particles incorporation in the adhesive system | Control (N0) | 50.31± 4.04 | A | ≤ 0.001* |
| Nanosilver Solution (N1) | 26.14± 4.16 | B | ||
| Nanosilver Powder (N2) | 47.72 ± 4.47 | A | ||
Self-etch adhesives exhibit limited antibacterial activity against Streptococcus mutans due to the presence of a low molecular weight monomer that possesses bacteriostatic action against Streptococcus mutans12.
The MIC for adhesive containing nanosilver in ethanol solution and powder was 12.5μg/ml concentration which was significantly different when compared to the control group (P≤0.05). These results confirmed the potential antibacterial effect of low concentrations of nanosilver. Adhesive resin with nanosilver powder at different concentrations showed significantly higher inhibition rates than that with nanosilver/ ethanol dispersion.
The higher significant efficiency of nanosilver powder may be attributed to the presence of the ethanol as a dispersion medium for nanosilver solution that can act as a diluting agent of the adhesive system. That was observed at concentration 6.25 mg/ml in nanosilver solution as it showed lower significant value than the control group despite the presence of silver nanoparticles.
The nanosilver powder group recorded a statistically non-significant difference in the degree of conversion when compared to the control group. Presence of nanofillers in the adhesive system had no harmful effect on the degree of conversion13.
Besides reducing of the amount of residual monomer, the nano-particles size is less than the wavelength of the blue light of the curing units that allow the passage of the light without scattering; thus doesn’t affect the degree of conversion and the depth of cure of the adhesive system14.
Groups of adhesive system with incorporated nanosilver solution recorded statistically significant lower degree of conversion when compared to the control and nanosilver powder (p≤0.05). Presence of excessive amount of ethanol (Over 10% of the neat resin blend) lead to dilution of the adhesive resin, (decrease the percent of polymerized resin). Moreover it can cause physical separation of some reactive components of the adhesive resin with subsequent reduction of the degree of conversion2. Previous research has attributed the negative effect of excess ethanol on the degree of conversion to its cooling effect (polymerization reaction is exothermic and the liberated heat increase the rate of conversion). Ethanol can absorb the liberated heat thus decrease the rate of polymerization and the degree of conversion of the adhesive15.
There were no recorded studies evaluated the effect of nanosilver (powder form or ethanol based solution) incorporation in the self-etch adhesive system on the degree of conversion.
The antibacterial efficacy of the adhesive system can be greatly potentiated with the addition of silver nanoparticles (12µg/mL concentration) especially the nanosilver powder. Incorporation of the antibacterial nanosilver powder in the adhesive system didn’t compromise the degree of conversion of the adhesive resin.
Further investigation is required for assessing the mechanical behavior and chemical reactions of adhesive systems containing silver nanoparticles in short and long-term. It is also recommended to evaluate the antibacterial activity of adhesive system containing silver nanoparticles against dental plaque biofilm rather than single bacterial species.
Open Science Framework: The effect of silver nanoparticles incorporation in the self-etch adhesive system on its antibacterial activity and degree of conversion: an In-vitro Study. https://doi.org/10.17605/OSF.IO/RS4D211
This project contains the following underlying data:
- Raw Data DC note pads (folder containing out files from ATR/FTIR)
- raw Data final DC.docx (ATR/FTIR data with explanation of analysis pipeline)
- results heba fathy.docx (inhibition zone measurements)
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nanotechnology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Shanmuganathan R, Karuppusamy I, Saravanan M, Muthukumar H, et al.: Synthesis of Silver Nanoparticles and their Biomedical Applications - A Comprehensive Review.Curr Pharm Des. 2019; 25 (24): 2650-2660 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Nanoparticles, Anticancer, Photocatalytic degradation; Cytotoxicity; Organic and inorganic Nanoparticles, Antibacterial
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 01 Mar 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)